Does a Hexagon Represent Carbon?
A hexagon does not represent a single carbon atom; instead, it represents six carbon atoms arranged in a cyclic structure, with hydrogen atoms implied to fulfill each carbon’s valence. This is central to understanding common representations in organic chemistry, especially in skeletal formulas.
Hexagon as a Representation of Multiple Carbons
When you see a hexagonal shape in organic chemistry diagrams, it typically depicts six carbon atoms connected in a ring. Each vertex of the hexagon corresponds to one carbon atom, collectively forming compounds like cyclohexane and benzene.
- Cyclohexane: a saturated ring with six carbons and associated hydrogens.
- Benzene: an aromatic ring of six carbons with alternating double bonds.
Understanding Skeletal Structures
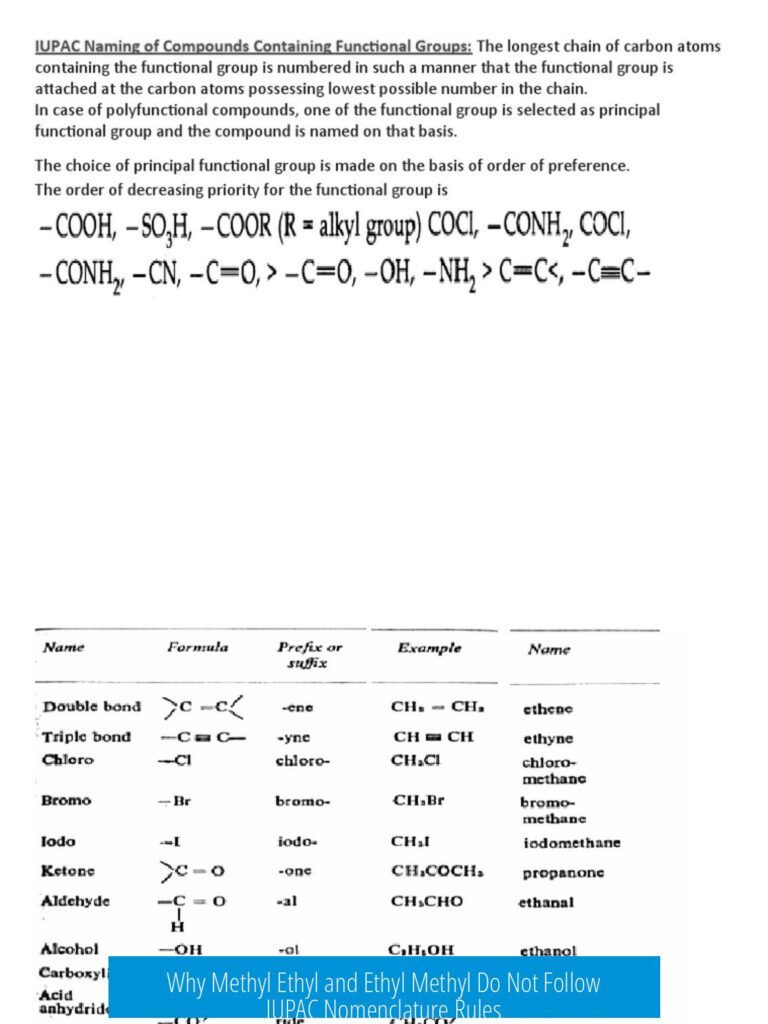
In skeletal (line-angle) structures, chemists omit showing carbons and hydrogens explicitly for simplicity. Instead:
- Each vertex (corner) of a line is a carbon atom.
- Lines between vertices indicate bonds—single, double, or triple.
- Hydrogen atoms bonded to carbons are usually not shown; they are implied to satisfy carbon’s tetravalency.
- Atoms other than carbon (oxygen, nitrogen, sulfur, metals) are always drawn explicitly.
This convention helps scientists quickly interpret molecular shapes and bonding without clutter. For example, a hexagonal skeletal structure with no other atoms drawn generally stands for a six-carbon ring.
Additional Notes on Simple Molecules
For simple molecules like methane (CH4), drawn as a dot or point in formulas, all hydrogens are explicitly considered, while larger molecules rely on implicit hydrogens.
| Aspect | Representation |
|---|---|
| Carbon Atom | Vertex in a line-angle/skeletal model |
| Hydrogen Atoms | Usually omitted unless attached to non-carbon atoms |
| Bonds | Lines between vertices; single/double/triple indicated by one, two, or three lines |
Key Takeaways
- A hexagon depicts a six-carbon ring, not a single carbon atom.
- Vertices in skeletal formulas represent carbon atoms.
- Hydrogens bonded to carbon atoms are usually not drawn but implied.
- Skeletal structures use lines for bonds, with heteroatoms shown explicitly.
- Simple molecules like methane may be represented differently, using dots or explicit formulas.



Leave a Comment