Do Periodic Table Stats Refer to Just One Atom of an Element?
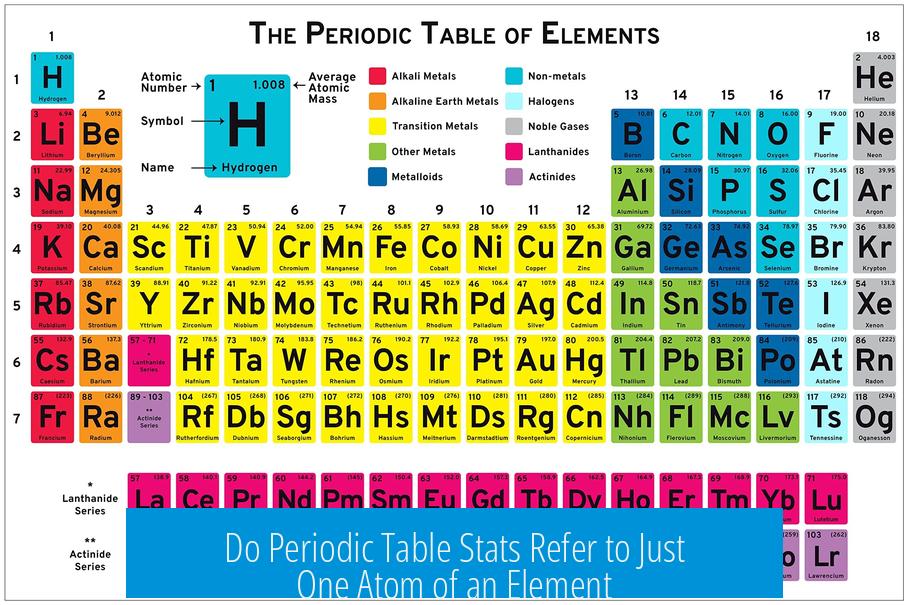
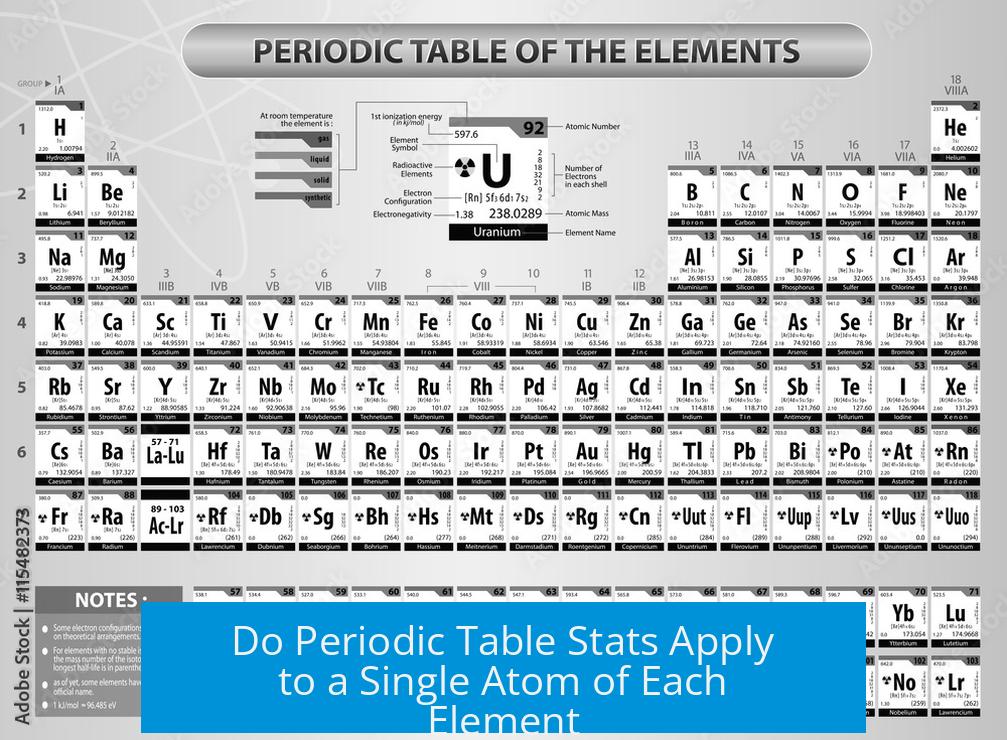
The values shown on the periodic table generally refer to the properties of a single atom of each element, such as atomic number and atomic weight. However, the atomic weight is an average value taking into account all the naturally occurring isotopes of that element rather than just one specific atom. Other properties like density or melting point relate to bulk forms composed of many atoms, often existing as molecules or lattices in nature.
Understanding Periodic Table Values Are Per Atom
The periodic table presents information primarily about individual atoms. For example:
- Atomic number indicates the number of protons in one atom’s nucleus.
- Atomic weight is an averaged mass of one atom, considering isotopes.
- Electron configuration represents the arrangement of electrons in a single atom.
This design reflects the fundamental nature of elements as defined by a unique proton count. Therefore, when you read the value for oxygen having an atomic number of 8, it means a single oxygen atom contains 8 protons.
Isotope Averaging and Atomic Weight
Atomic weight values do not correspond to any one atom precisely because most elements exist as a mixture of isotopes. Each isotope has a slightly different mass due to differing neutrons.
For example, natural chlorine comprises about 75% chlorine-35 atoms and 25% chlorine-37 atoms, so its atomic weight is roughly 35.45 atomic mass units (amu), a weighted average across isotopes.
Thus, no single chlorine atom weighs exactly 35.45 amu. This weighted average is what the periodic table lists as the atomic weight for chlorine.
Element vs Atom vs Elemental Forms
An important distinction is between the terms “atom” and “element.”
- Atom: The smallest particle of an element, defined by its proton count.
- Element: A substance composed of atoms all sharing the same number of protons.
- Elemental forms: These are physical manifestations of the element, which may be single atoms, molecules made of multiple atoms of the same element, or extended networks.
Examples of Elemental Forms
- Noble gases (e.g., helium, neon) exist naturally as single atoms.
- Non-metal molecules: Oxygen commonly exists as O2 molecules, sulfur as S8 ring molecules.
- Metal lattices: Metals like iron or copper form large lattices of atoms bonded together.
Therefore, the periodic table data refer to a single atom’s properties. But elements generally exist in nature as molecules or networks formed by those atoms.
Atoms Alone Do Not Equal Elements in Nature
You rarely find isolated single atoms of elements like oxygen or hydrogen outside lab settings. They quickly form molecules such as O2 or H2, which are stable elemental forms. This is because isolated reactive atoms seek to bond for stability.
Some elements like the noble gases exist as single atoms naturally due to chemical inertness. Yet, these exceptions do not change the periodic table’s atomic focus.
Bulk Properties Come from Many Atoms or Molecules
Properties such as melting point, boiling point, density, and half-life depend on physical forms composed of many atoms. The periodic table sometimes includes these values, but they are not intrinsic to single atoms.
| Property | Applies To | Example |
|---|---|---|
| Atomic Mass | Single atom (average isotopes) | Oxygen atom mass ≈ 15.999 amu |
| Melting Point | Bulk material or molecule | O2 boils at -183 °C |
| Density | Bulk form | Solid iron density ≈ 7.87 g/cm3 |
Thus, such additional stats reflect properties of elemental forms made up of many atoms. Atomic or molecular arrangements influence these features.
Clarifications on Molecular and Ionic States
While the periodic table focuses on atoms, elements can form molecules or ions during chemical reactions.
- Molecular elements like O2 or S8 consist only of one element’s atoms.
- The element itself is defined by atomic structure, not molecular grouping.
- When compounds ionize, atoms become charged and interact differently, e.g., NaCl dissociates into Na+ and Cl− ions.
Key Points on Periodic Table Data and Elements
- Periodic table stats mainly describe properties of single neutral atoms, especially atomic number and atomic weight.
- Atomic weight is a weighted average of isotopes, not the mass of a single atom.
- Elements can exist as atoms, molecules, or extended structures in nature.
- Most elements naturally form molecules or lattices, not free single atoms.
- Bulk properties on the table apply to grouped atoms or molecules rather than individual atoms.
- Molecular elemental forms (like O2) differ from the atomic definition but still represent the same element.
1. Does the periodic table show data for a single atom of each element?
Yes, the periodic table lists data referring to one atom of an element. For example, oxygen’s atomic number is for a single oxygen atom. However, some properties like atomic weight are averaged over natural isotopes.
2. Why is the atomic weight on the periodic table not a whole number?
Atomic weight is a weighted average of all naturally occurring isotopes of that element. For chlorine, for example, it averages isotopes with masses 35 and 37 AMU, giving a value like 35.45 AMU, which no single atom has exactly.
3. Are elements always found as single atoms in nature?
Many elements, like noble gases, exist as single atoms naturally. Others, like oxygen or sulfur, usually form molecules (O2, S8) or networks. Single atoms of these elements rarely exist isolated due to their reactivity.
4. Does the periodic table reflect properties of elemental molecules like O2 or S8?
No, the periodic table primarily reflects individual atom properties. Bulk properties like melting point or density relate to elemental molecules or macroscopic forms, not single atoms.
5. Can an element be a molecule or a network of atoms?
Yes. Elements can form molecules (O2, P4) or extended networks (diamond carbon). Still, the element is defined by proton count. The periodic table focuses on one atom’s stats, not these molecular forms.



Leave a Comment