Electroplating with Silver: Comprehensive Insights and Practical Guidelines
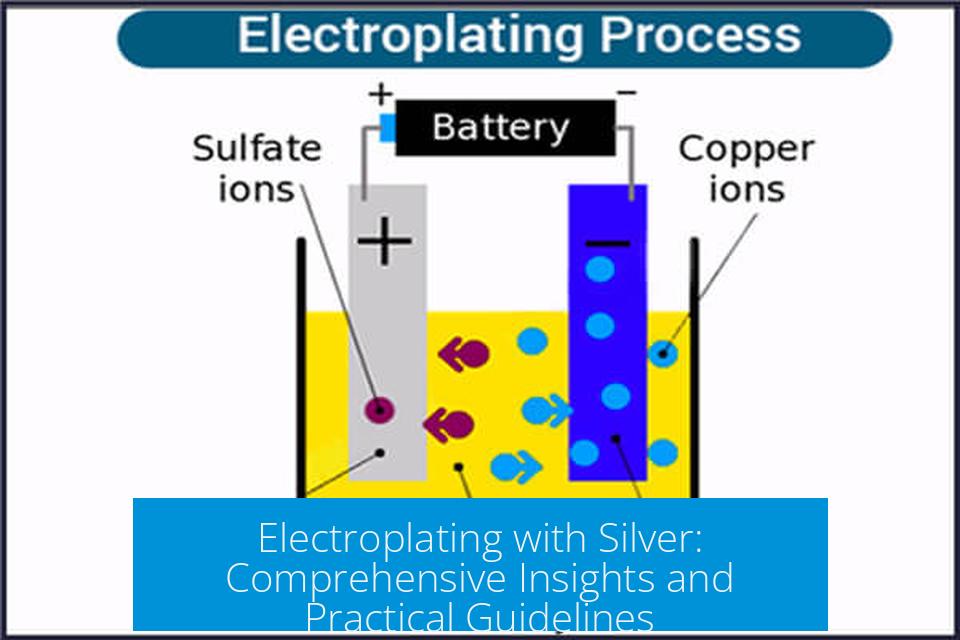
Electroplating with silver involves depositing a thin layer of silver onto a conductive object using an electrical current. The process requires carefully selected chemistry, precise electrical control, and proper preparation to achieve high-quality and durable silver coatings. This article examines silver electroplating’s core principles, addressing common challenges, industry standards, safer alternatives, and practical recommendations for beginners and professionals.
1. Challenges of Using Silver Nitrate in Electroplating
Poor Quality Deposits from Silver Nitrate
Silver nitrate solutions are frequently used in various silver chemistry applications. However, they are unsuitable for producing hard, adherent silver deposits via electroplating. The silver layer obtained tends to be spongy and loosely bound.
This spongy structure results from uneven deposition and weak adhesion. Thus, attempts to plate silver using silver nitrate commonly yield a crumbly, porous silver layer rather than a solid metallic finish.
Influence of Electrical Parameters on Silver Nitrate Plating
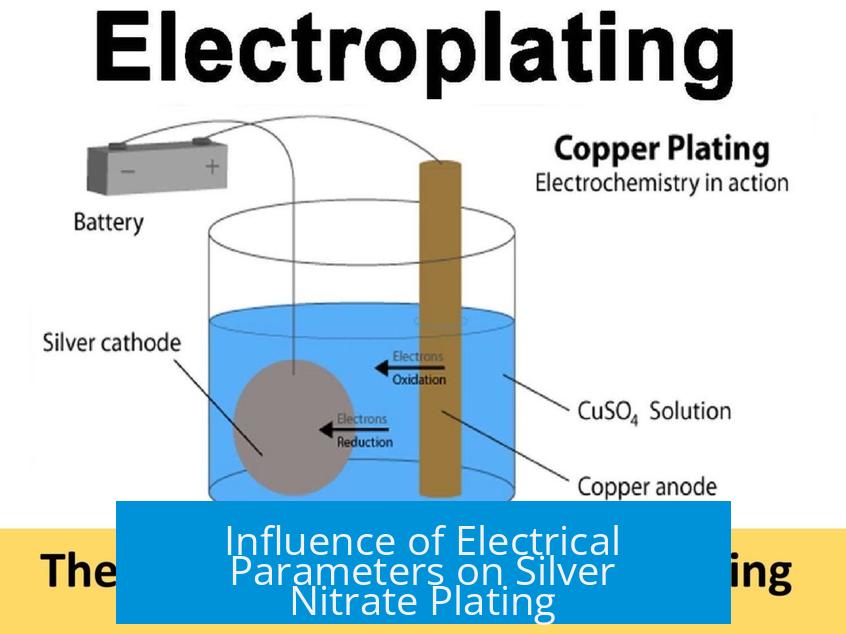
Voltage and current density require precise control. The reduction potential for silver deposition is approximately 0.8 volts. Applying voltage beyond this, or excessive current, causes poor deposition quality. High overpotential speeds up silver ion reduction but encourages rough, non-coherent deposits.
Many plating failures result from using power supplies delivering voltages higher than 0.8 volts or currents beyond the optimal range, or from overly concentrated silver nitrate baths. Precise monitoring of both voltage and current density is essential.
Additional Factors Affecting Silver Nitrate Plating
- Plating bath concentration: Ideal silver nitrate concentration often lies within a narrow window to ensure smooth deposition.
- Temperature: Electroplating performance depends on bath temperature; deviations can cause irregular deposits or increased gas evolution.
- Gas evolution: Bubbling or gas formation on electrodes indicates side reactions, reducing plating efficiency.
2. Industry-Standard Silver Plating Chemistry
Silver Cyanide Baths for Professional Plating
The industrial standard for silver plating employs silver cyanide-based solutions enhanced with potassium cyanide. These baths provide consistent deposition, excellent adhesion, and bright shiny finishes. Cyanide complexes improve plating thickness uniformity and current efficiency.
However, cyanide compounds are highly toxic and require specialized handling and disposal procedures. This toxicity restricts their use to trained industrial environments with proper safety equipment and protocols.
Safer Alternative Silver Plating Chemistries
In response to cyanide toxicity, safer alternatives have been developed. These include baths based on:
- Thiosulfate
- Ammonia
- Sulfite
- Succinamide
- Thiourea
Each alternative offers reduced toxicity but comes with limitations. These chemistries operate well only within narrow pH, temperature, and concentration ranges. Moreover, they often require additives to enhance plating quality and achieve desired properties such as brightness and hardness.
3. Recommendations for Demonstrations and Beginners
Using Commercial Silver Plating Solutions
For those new to silver electroplating or aiming to demonstrate the process, buying a quality commercial silver plating solution is advisable. Although more costly, these solutions are optimized for stability, consistent plating characteristics, and often contain additives to improve adhesion and brightness.
Non-cyanide-based commercial solutions are available, offering safer handling. Strictly following the manufacturer’s instructions is key to success with these products.
Advice on Personal Silver Plating Attempts
Silver plating involves complex chemistry and precise electrical control. Individuals seeking a silver-plated object may find it simpler and more reliable to use professional plating services. These businesses have optimized baths, proper equipment, and safety protocols.
Attempting silver plating without experience can lead to poor plating quality, equipment damage, or chemical hazards. Personal plating attempts require a learning curve, thorough safety precautions, and acceptance of potential failures.
Working with acids and toxic chemicals necessitates proper gloves, ventilation, and adherence to protocols. Exposure to acids or contamination poses health risks easily avoided with appropriate preparation.
4. Key Process Details and Additives for Quality Plating
Substrate Cleaning and Preparation
Quality silver plating starts with a clean substrate. Thorough removal of oxides, grease, and contaminants is essential to encourage good adhesion. Common procedures include:
- Immersion in acid or mechanical polishing to remove surface oxides
- Rinsing in solvents like acetone to eliminate oils
- Ensuring the substrate is dry and handled with clean tools
Even small residues or surface irregularities can cause plating defects or flaking.
Additives for Improved Plating Performance
The use of additives helps control deposit morphology, brightness, and adhesion. For example, thiosulfate-based baths benefit from:
- Brighteners—organic compounds that promote smooth, shiny deposits
- Surfactants—reduce surface tension and improve bath wettability
- Simple additives like sugars (e.g., 6 grams of table sugar per liter) can enhance deposit texture and brightness
- Commercial drying agents such as Jet Dry improve surface finish by reducing water spots
5. Setup and Electrical Parameters for Successful Plating
Voltage and Amperage Control
Silver reduction requires around 0.8 volts. Applying significantly more voltage risks poor deposits and hydrogen evolution at the cathode, leading to rough or spongy silver layers. Amperage must match the surface area of the object: high current density can cause uneven plating, while too low current yields slow or incomplete coatings.
Power sources such as low-voltage batteries or regulated power supplies are suitable. For example, simple “Baghdad battery” setups provide about half a volt and low amperage suited for decorative or experimental plating.
Wiring Configurations
Electroplating circuits can be wired in series or parallel, depending on plating multiple objects or scaling up. There are no secret configurations beyond standard electrical wiring principles:
- Series wiring: Same current flows through all objects, useful for consistent current distribution.
- Parallel wiring: Same voltage is applied across all objects, current divides based on object resistance.
The choice depends on the power supply capacity and the number or size of objects plated.
6. Safety Considerations and Toxicity
Toxicity of Cyanide-Based Baths and Chemical Hazards
Cyanide-based silver plating baths pose severe health risks. Cyanides are acutely toxic, and accidental exposure can be fatal. These solutions require strict handling with gloves, protective clothing, and fume extraction.
Acids used in surface preparation or plating baths are corrosive and cause burns. Proper protocols include working in well-ventilated areas, wearing safety goggles, and using chemical-resistant gloves.
Ignoring safety procedures leads to chemical burns, respiratory issues, or long-term health hazards.
7. Additional Observations and Ethical Comments
Substrate Material Verification
Before plating, verify the substrate metal. For example, coins are often copper-plated zinc rather than solid copper. Such composite substrates can affect adhesion or produce uneven plating. Chemical or visual tests can confirm substrate composition.
Knowledge Sharing and Industry Transparency
Electroplating techniques have historically been protected by industry secrecy. Sharing detailed plating knowledge encourages safer, more effective methods and counters exploitation by less knowledgeable users.
Ethical science promotes open access to verified procedures to help individuals learn and improve without risking health or wasting materials.
Summary of Key Points
- Silver nitrate plating yields poor-quality, spongy silver deposits unsuitable for durable applications.
- Industrial silver plating uses cyanide-based baths for consistent results but poses toxicity risks.
- Safer alternatives involve complex chemistries (thiosulfate, sulfite) requiring precise control.
- Beginners should consider commercial plating solutions or professional services to avoid hazards and failure.
- Proper cleaning and substrate preparation significantly influence plating quality.
- Voltage around 0.8 V and controlled amperage are critical to prevent rough deposits and hydrogen evolution.
- Use of additives like brighteners and surfactants improves deposit appearance and adhesion.
- Strict safety precautions are mandatory when handling acids and toxic compounds.
Electroplating with Silver: What You Need to Know Before You Dip That Coin
Electroplating with silver might seem like magic — you dunk a dull object in a solution, apply a bit of current, and suddenly you have a shiny silver finish. But before you get your hopes up and splash your pennies, here’s a reality check: silver nitrate is NOT your friend if you want a strong, smooth, and durable silver coating. Let’s unravel this fascinating yet tricky process.
First off, why not just use silver nitrate? You might have read it’s a common silver compound and assumed it would be perfect for plating. Brace yourself: plating with silver nitrate typically produces a spongy, crumbly silver layer. Not exactly the elegant mirror finish you want. This deposit lacks strength and adherence, meaning it falls apart easily. Imagine plating your precious coin only to watch the silver peel away like the worst kind of sticker shock.
Why is this happening? Essentially, silver nitrate’s plating conditions are tough to control. Voltage, current, temperature — all need to be just right. You see, silver ions naturally reduce at about 0.8 volts. Push more than that, and you’re inviting trouble: poor deposit quality and unwanted side effects like gas bubbles forming on your electrode. Ever had bubbles block your view while cleaning dishes? Now imagine bubbles messing up your precious silver layer. Not a happy scene.
Even the concentration of your silver nitrate solution matters. Run your voltage too high on your power supply reading 2 volts, and you’re likely making a mess, not magic.
Industry Doesn’t Mess Around: Cyanide Baths Rule
If you think silver nitrate is tricky, the industry standard definitely cranks the difficulty up a notch — with silver cyanide-based plating baths. Yes, you read that right: cyanide. These baths use potassium cyanide for better electrical characteristics, yielding silver coatings of stellar quality. But hold your horses before mixing up any cyanide bath at home! These chemicals are viciously toxic, not something a curious hobbyist should handle. Cyanide plating is for pros with extensive safety gear and training.
For those concerned about safety, enthusiasts have developed alternatives. Safer chemistries use substances like thiosulfate, ammonia, sulfite, succinimide, and even thiourea. These options mitigate some toxicity but bring their own headaches: you must control pH, temperature, current density, and concentrations to a tee. Step out of the very narrow comfort zone, and you risk weak deposits or no plating at all.
Beginners, Here’s Your Cheat Sheet
Want to try silver plating — but don’t want to risk becoming a chemistry casualty? Your best bet is to grab a commercially available silver plating solution. Yes, they’re pricey, but they handle all the complicated balancing acts for you. Some even come non-cyanide based if you look hard enough. Follow those instructions like they’re the recipe to grandma’s secret sauce. Setup? Follow wiring suggestions, whether in series or parallel. Remember, wiring isn’t rocket science. Think: two roads, not a maze.
If simply having a silver-plated object is your goal, an even easier option is to send it to a silver plating business. They’re pros and can deliver great results. Honestly, silver plating has a steep learning curve. Don’t dismiss the advice to tread carefully — unless you’re in it for the adventure of learning and the occasional frustrating trial.
The Hidden Details That Make or Break Your Silver Plating
Before the plating begins, your metal object needs thorough cleaning. Oxide layers, grease, and dirt are plating’s worst enemies. Imagine trying to glue two surfaces together with dust between them; nothing sticks. The coin, for instance, must be cleaned, stripped of oxide, and washed in acetone. This prep work makes silver happily stick instead of flaking off.
Now, the secret ingredients to get a shiny, bright, and smooth surface: additives. Commercial setups often include brighteners and surfactants to make silver deposits look like that high-polish finish you admire in jewelry stores. For DIY fans, adding 6 grams of table sugar per liter of plating solution and some commercial “jet dry” surfactant can help. Why sugar? It acts in a surprising way to brighten plating — fascinating chemistry at work!
Voltage and Amperage: The Power Struggle
Electricity powers plating, but dose it wrong and you’re either wasting time or ruining your work. Remember, silver’s reduction potential is roughly 0.8 volts. Exceeding this with high voltage or high amperage can cause spongy deposits or gas bubbles. Fun fact: those mythical Baghdad batteries from ancient times put out about half a volt, just about enough to start chemical reactions but not enough for quality silver plating.
Safety First! This Isn’t a Science Fair Project
Please, don’t get reckless. You’re mixing acids, toxic chemicals, and electricity. Skin contact with acid can be dangerous; if you feel burning, it might already be too late. Wear proper gloves, goggles, and work in a ventilated area. When in doubt, don’t do it alone — this isn’t the time for “science by osmosis.” Follow all safety protocols religiously. Your shiny project isn’t worth your health or worse.
Are You Sure About Your Metal?
One last pro tip: know your substrate. Electroplating copper is common, but many coins and objects are copper-plated zinc or other metals, which behave differently. Trying to plate over the wrong metal can leave you with unpredictable or ugly results. Do a quick metal test before starting — it pays off.
Sharing Knowledge: A Silver Lining
Now, here comes the juicy part. The dense, secretive world of electroplating is often shrouded by “trade secrets” and proprietary formulas. But transparency comes with power. Sharing real info, even about toxic cyanide baths or tricky thiosulfate chemistry, helps newcomers avoid disasters and pushes the craft forward. It’s like revealing the magician’s trick — minus the smoke and mirrors.
“I JUST GAVE AWAY ALL OF THESE CON ARTISTS, SCHEMING PEOPLE, AND MOST SICKENING OF ALL GREEDY SELFISH CORRUPTERS’ SECRETS!!!”
Knowledge is power, especially when it’s free and shared with care.
Final Sparkling Thoughts
Electroplating with silver is an intriguing blend of science and art. Don’t expect to succeed on your first try, especially if relying on silver nitrate—it’s more of a spongy headache producer than a finishing tool. The industry’s use of cyanide baths clearly illustrates what works best, albeit at a cost to safety. Safer alternatives exist but demand precise conditions and chemistry juggling.
If you’re a beginner, grab a commercial plating kit and follow the directions. Or, want something shiny without grief? Ask a professional. The magic of silver plating hides behind years of knowledge, careful prep, controlled electrical conditions, and yes, strict safety measures.
So next time you see a beautifully plated silver piece, appreciate the science, skill, and safety effort it took to achieve that gleam. And if you try plating yourself, may your surfaces be shiny and your fingers safe — because sometimes, the best silver lining is the one you don’t have to scrape off later.
What problems occur when using silver nitrate for electroplating?
Silver nitrate produces poor quality silver deposits. The plated silver often turns out spongy and crumbly. It does not form a hard, adherent layer suitable for most applications.
Why is silver cyanide preferred in industrial silver plating baths?
Silver cyanide offers better current characteristics and produces hard, durable silver coatings. It’s the industry standard despite being highly toxic and requiring expert handling.
Are there safer alternatives to cyanide-based silver plating solutions?
Yes, solutions based on thiosulfate, ammonia, sulfite, and thiourea exist. However, these require precise control of pH, concentration, and temperature for good results.
What are key factors for successful silver plating at home?
Clean the substrate thoroughly to remove oxides. Use a commercial plating solution and control voltage and current to about 0.8 volts to avoid poor deposits.
How does voltage affect silver plating quality?
Too high voltage or current causes weak, spongy deposits. Silver plating works best near its natural reduction potential of 0.8 volts.
Is DIY silver plating recommended for beginners?
Due to toxic chemicals and complex conditions, beginners should consider using commercial kits or professional services. Silver plating involves risks and a learning curve.



Leave a Comment