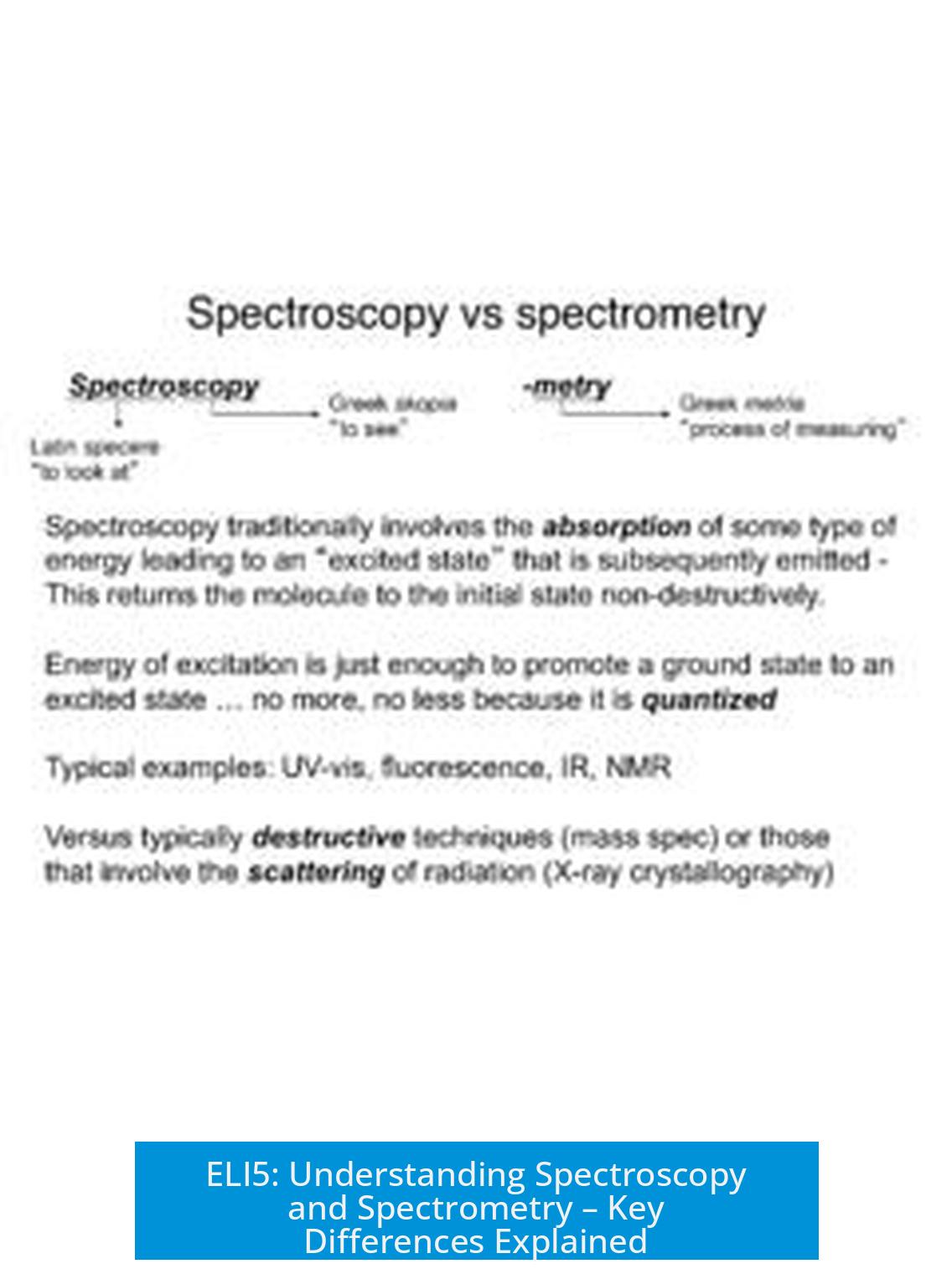
ELI5: Spectroscopy vs Spectrometry

Spectroscopy is the study of how light or electromagnetic radiation interacts with matter, while spectrometry is the measurement or quantification of those interactions. These terms are related but differ in focus and application.
Understanding Spectroscopy
Spectroscopy involves examining how particles absorb, emit, or scatter electromagnetic energy. It is a broad field exploring the entire spectrum of radiant energy. The primary aim is to observe the interaction between radiation (like light) and matter.
- Focus: Absorption or emission of electromagnetic waves by particles.
- Scope: Studies entire spectrum behavior, including transmission through a medium.
- Example: UV-Vis spectroscopy studies how ultraviolet and visible light are absorbed by molecules.
Defining Spectrometry
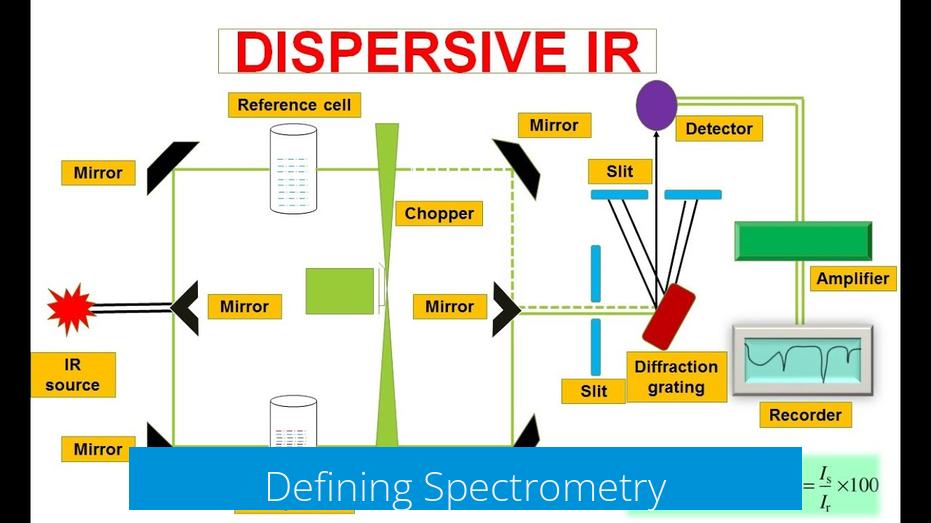
Spectrometry focuses on measuring these spectroscopic phenomena, often quantifying the amount of energy absorbed or emitted. It also includes counting particles, such as ions in mass spectrometry (MS).
- Focus: Precise measurement of energy at specific wavelengths or mass-to-charge ratios.
- Instrument: A spectrometer detects and measures intensity at chosen wavelengths or masses.
- Example: Mass spectrometry quantifies ions based on their mass and charge.
Key Differences Summarized
| Aspect | Spectroscopy | Spectrometry |
|---|---|---|
| Definition | Study of interaction between radiation and matter. | Measurement of specific spectroscopic properties or particles. |
| Focus | Absorption, emission, or scattering of electromagnetic energy. | Quantification of wavelengths, energies, or ion counts. |
| Scope | Broad investigation of spectra. | Specific measurement at defined points or mass/energy ranges. |
| Example Techniques | UV-Vis, infrared spectroscopy. | Mass spectrometry, spectrophotometry. |
Common Confusions

People often use spectroscopy and spectrometry interchangeably. However, a spectroscope scans a spectrum across multiple wavelengths or energies, while a spectrometer measures intensity at a single or narrow range. Spectrometry is effectively the practical, quantitative application of spectroscopy.
Source Authority
The International Union of Pure and Applied Chemistry (IUPAC) clarifies these terms in the Gold Book. For additional insights, LSU provides a detailed explanation of their distinctions.
Key Takeaways

- Spectroscopy studies how electromagnetic energy interacts with matter.
- Spectrometry measures or quantifies those interactions precisely.
- Spectroscopy covers broad spectral analysis; spectrometry focuses on specific measurable data.
- Mass spectrometry counts ions; spectrophotometry measures light absorption.
- The two terms are related but not identical; correct use aids clarity in scientific communication.
ELI5: Spectroscopy vs Spectrometry — What’s the Real Difference?
So, you’ve stumbled upon the terms spectroscopy and spectrometry and you’re scratching your head wondering: Are these just fancy science jargon for the same thing? Or do they actually mean different stuff? Well, buckle up — we’re about to unravel this scientific duo in simple terms, with a light sprinkle of fun!
In a nutshell: Spectroscopy is the study of how light or electromagnetic radiation interacts with matter. Spectrometry, on the other hand, is the measurement of those interactions — like turning research into tangible numbers. Put simply: spectroscopy = studying the spectrum; spectrometry = measuring the spectrum.
The Tale of Two Science Words
Let’s start with the word roots. “Spectro” means spectrum—think of a rainbow or light split into its colors, spread out like a slick science show. “Scopy” means study—so spectroscopy means the study of how these spectra behave. “Metry” means measure, so spectrometry is all about measuring those spectra more precisely.
Here’s a neat analogy. Suppose you’re at a music festival. Spectroscopy would be like being the curious listener who tries to figure out how the sound waves from different instruments mingle and interact. Spectrometry? That’s the techie behind the scenes holding the device that measures the exact frequency and intensity of the sound waves coming from each instrument.
Spectroscopy: The Broad Curiosity
Spectroscopy looks at how many different particles (atoms, molecules, or ions) behave when light or other forms of electromagnetic radiation hit them. Does the particle absorb the light? Reflect it? Emit some? How does that change depending on the light’s wavelength or energy?
It covers a wide range—from visible light to X-rays, radio waves, or even gamma rays. Scientists study these interactions to understand what something is made of or to analyze its properties. For example, astronomers use spectroscopy to decode the composition of stars—what’s that glowing ball made of, anyway?
Spectrometry: The Precision Operator
Spectrometry is about using instruments, aka spectrometers, to measure specific parts of the spectrum. Imagine your spectrometer as a meticulous scanner that isolates a specific wavelength or energy level. It results in a spectrum you can analyze quantitatively.
In mass spectrometry (a big name in chemistry labs), instead of focusing on light absorption, the device counts particles like ions and sorts them by their mass-to-charge ratio (m/z). This is crucial for figuring out the exact molecular structure of a compound. It’s the superhero tool for chemists who want to know “what’s in the mixture?”
Simply put, spectrometry is the practical application—grabbing the abstract data of how things absorb or emit energy and making it measurable and useful.
Why the Confusion?
- People often use spectroscopy and spectrometry like interchangeable twins, but they’re not quite the same.
- A spectroscope scans or observes a range of wavelengths (think of it as the curious observer), while a spectrometer measures exact wavelengths or energies (the detail-oriented nerd).
- Sometimes spectrometry is referred to as “using spectroscopy to quantify something.”
The International Union of Pure and Applied Chemistry (IUPAC Gold Book) codifies these differences officially, but chatting about it casually gets a bit muddled—especially since multiple scientific disciplines and instruments might overlap.
The Practical Impact: Why Should You Care?
Still wondering why this distinction matters? Well, here’s a simple example:
Imagine you work in food safety. Spectroscopy might help you understand what kinds of compounds exist in your spinach leaves by studying how they interact with light. Spectrometry allows you to measure their quantities precisely—like how much pesticide residue is present or how much vitamin K is packed inside.
In medicine, spectroscopy can help understand biochemical changes in tissue by how light interacts with it; spectrometry can count biomolecules precisely, guiding diagnoses.
How Do These Techniques Show Up in Real Life?
Plenty of everyday technologies rely on these concepts:
- Spectroscopy: Used in astronomy to analyze the light from stars and planets, helping discover new worlds.
- Spectrometry: A tool in environmental monitoring, where mass spectrometry measures pollutants at extremely low concentrations.
- Pharmaceuticals use spectrometry to ensure drugs are pure and dosed right.
A Summary Table to Crystalize Things
| Aspect | Spectroscopy | Spectrometry |
|---|---|---|
| Primary Focus | Study of how electromagnetic radiation interacts with matter | Measurement of those interactions (intensity, wavelength, particle count) |
| Nature of Activity | Broad, observational, qualitative | Precise, quantitative, specific |
| Examples | Absorption spectroscopy, emission spectroscopy | Mass spectrometry, spectrophotometry |
| Device | Spectroscope (scans multiple wavelengths) | Spectrometer (measures a single or set of wavelengths) |
| Etymology | “Spectrum study” | “Spectrum measure” |
Wrap-Up: The Double Act You Need to Know
In the simplest terms possible — spectroscopy is the curious scientist looking carefully at the light-matter dance, while spectrometry is the number-crunching technician turning observations into data. They work hand-in-hand. Without spectroscopy, we wouldn’t know what’s worth measuring. Without spectrometry, we wouldn’t have precise information to act upon.
Next time you hear about spectroscopy and spectrometry in a lab or a documentary, you’ll know exactly how to impress your friends — or at least keep your curiosity satisfied.
Now, what about you? Have you encountered these terms in your studies or work? Or maybe even in sci-fi movies? Drop your thoughts, and let’s make this spectrum of understanding even broader!
What is the main difference between spectroscopy and spectrometry?
Spectroscopy is the study of how light or electromagnetic energy interacts with matter. Spectrometry is the measurement of these interactions, often quantifying absorbed energy or counting particles.
Can spectroscopy and spectrometry be used interchangeably?
They are often confused but not the same. Spectroscopy studies multiple wavelengths. Spectrometry measures specific wavelengths or particle counts.
How does a spectrometer differ from a spectroscope?
A spectroscope scans different wavelengths or energies. A spectrometer measures one specific wavelength or energy at a time.
Is spectrometry only about light absorption?
No. Spectrometry includes measuring light absorption but also counting particles like ions, as in mass spectrometry.
Why do we say “spectrum study” for spectroscopy and “spectrum measure” for spectrometry?
The suffix “-scopy” means study, so spectroscopy means studying spectra. The suffix “-metry” means measure, so spectrometry means measuring spectra.




Leave a Comment