Understanding Emission Spectrum

The emission spectrum reveals the presence of specific elements by displaying the distinct wavelengths of light those elements emit when energized. This principle allows scientists to determine chemical compositions, even of distant celestial bodies, by analyzing the light they emit.
How Emission Spectra Identify Elements
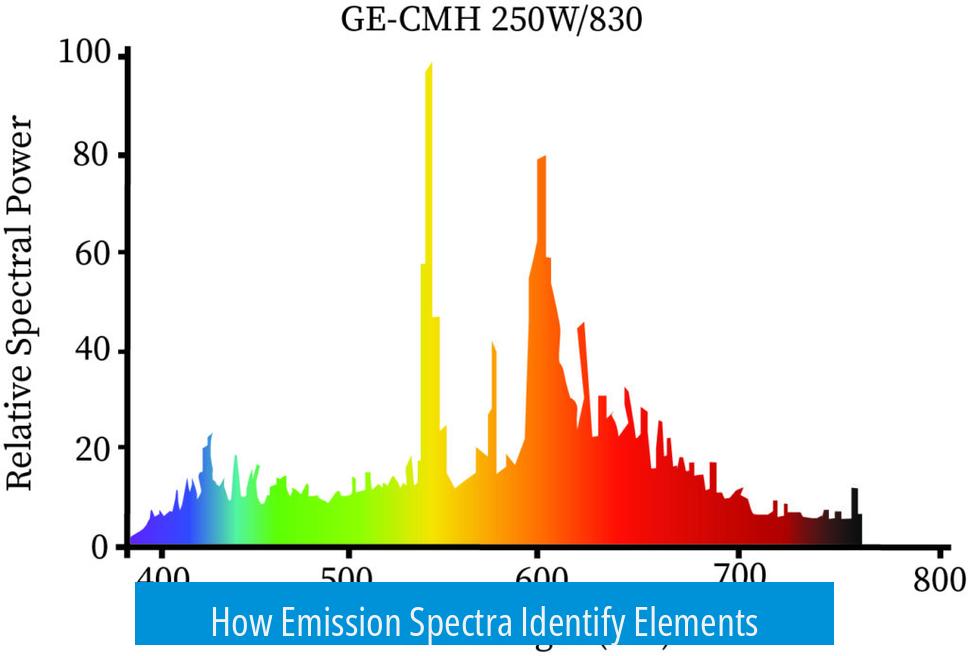
Each element emits light at specific wavelengths, producing unique spectral lines. When an element’s atoms get excited, electrons jump to higher energy levels. Upon returning to their original states, they release photons at characteristic energies. These create a pattern of bright lines, an emission spectrum, acting as a fingerprint for that element.
Application in Astronomy
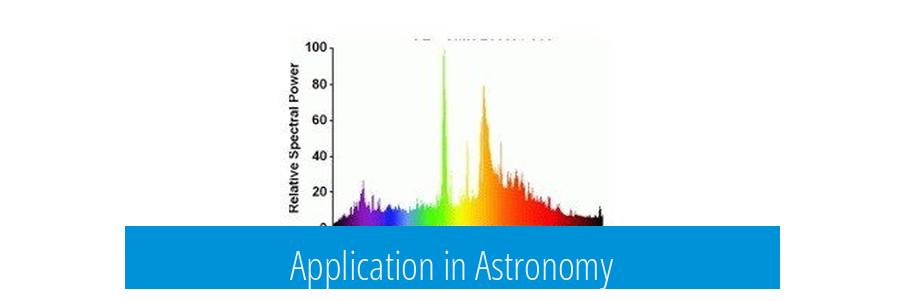
Astronomers analyze light from stars and gas clouds. By splitting this light into its emission spectrum, they can identify what elements are present. This method helps determine the composition of celestial bodies without direct sampling.
Challenges with Unknown or Mixed Gases
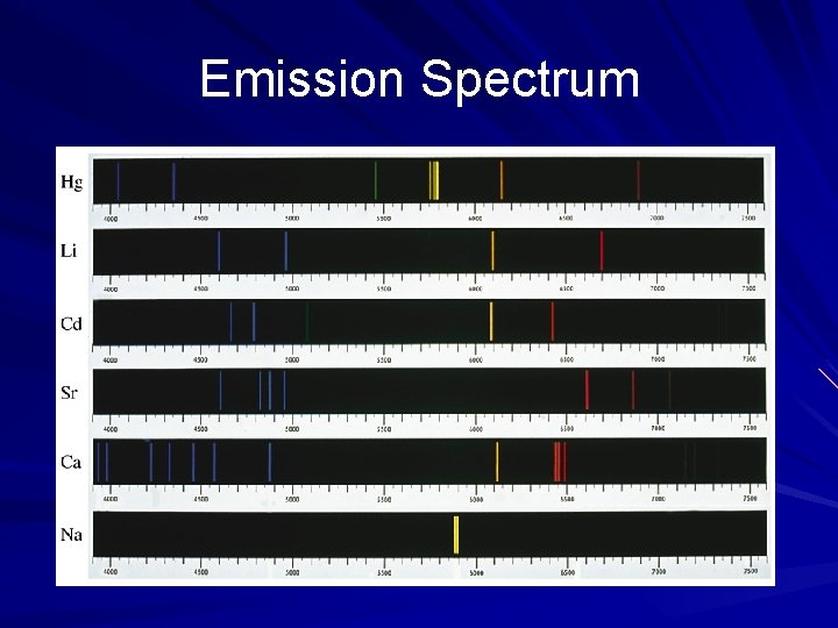
- Unknown gases may produce emission spectra that resemble known elements initially but differ upon detailed examination.
- Mixed gases create complicated spectra with overlapping lines from multiple elements.
- Careful comparison of spectral lines is essential to avoid misidentification, as some lines may coincide by chance.
Case Study: Comparing Unknown Gas to Helium
An unknown gas shows emission lines superficially similar to helium’s spectrum. Closer inspection reveals differences, suggesting the presence of other components besides helium. This scenario underscores the difficulty of interpreting spectra from mixtures and the need for precise spectral analysis.
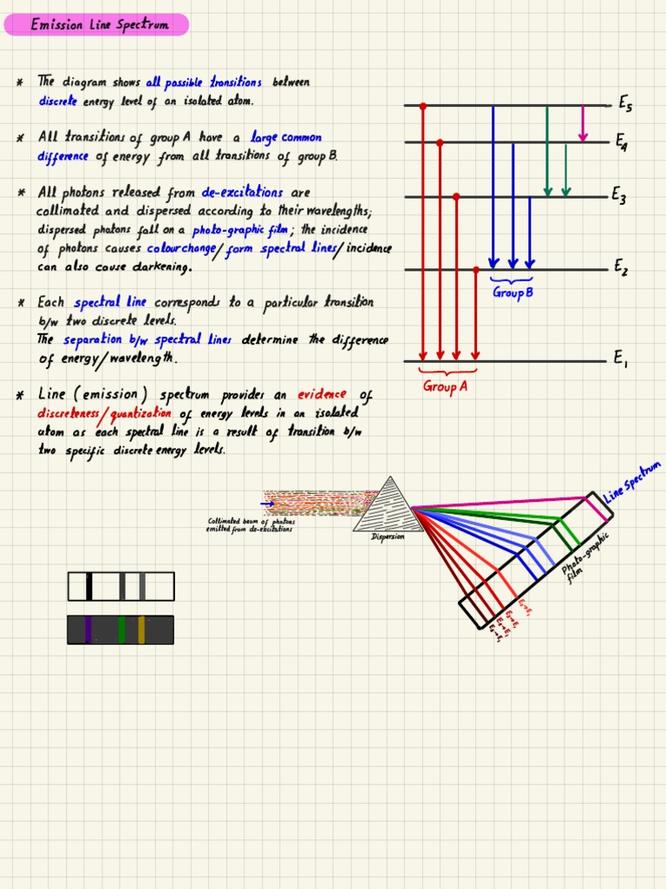
Summary of Key Points
- Emission spectra provide direct evidence of the elements present in a sample.
- Each element’s spectrum is unique, allowing for accurate identification.
- Mixtures complicate analysis due to overlapping spectral lines.
- Differentiating elements in unknown gases requires detailed spectral comparison.
- Emission spectroscopy is critical in astrophysics for studying chemical compositions remotely.
Emission Spectrum: The Cosmic Detective’s Secret Weapon
Ever wonder how scientists figure out what stars, nebulae, or distant galaxies are made of? The answer is hidden in light — specifically, in the emission spectrum. This brilliant tool acts like a cosmic barcode reader, revealing the elements present in celestial bodies by analyzing the specific wavelengths of light they emit.
But this story is not as straightforward as shining a flashlight and naming the colors that come out. Sometimes, light can be sneaky, especially when we try to decode gases we don’t fully understand. Let’s embark on a lively journey through the science of emission spectra and uncover why this technique is a game-changer — and occasionally, a head-scratcher.
What is an Emission Spectrum, Anyway?
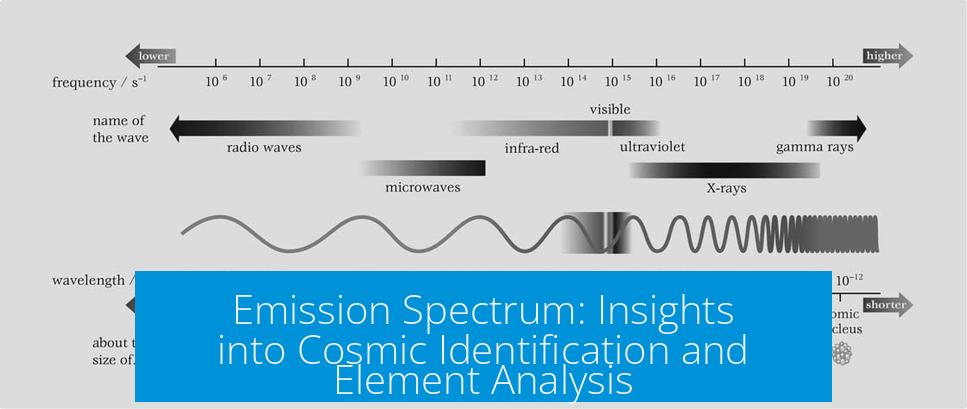
Think of an emission spectrum as a fingerprint made of light. When an atom or molecule gets excited — say, by heat or electricity — it releases light at very specific wavelengths unique to that element. These bright lines or bands appear when we pass the light through a prism or diffraction grating, separating it into its component colors.
For instance, helium, the noble gas most famous for lighter-than-air balloons, glows with a very distinct set of lines in the visible spectrum. Each element boasts a unique pattern, allowing scientists to identify them in distant stars or gases here on Earth.
Using Emission Spectra to Identify Celestial Elements
Here’s where it gets truly fascinating. Astronomers can’t send probes to most stars or nebulae. Instead, they study the light reaching Earth. By analyzing the emission spectra of that light, researchers identify what those distant objects contain.
For example, a star’s spectrum may show strong hydrogen lines mixed with subtle traces of calcium or iron. That’s how scientists unlock stellar secrets — without leaving their labs.
This method is so reliable it revolutionized astronomy, enabling discoveries about the universe’s makeup and evolution. Sounds impressive? It is!
The Mystery of the ‘Unknown Gas’ Spectrum
But wait. What if the spectrum you get looks like helium’s but might not be helium? That’s a real puzzle scientists face when dealing with unknown gases.
Imagine you have a sample labeled “unknown gas.” The emission spectrum shows a few bright lines that match helium’s signature at first glance. Tempting to jump to conclusions, right? But a closer look reveals subtle discrepancies. Some lines may be missing, some extra lines appear, and overall, the match isn’t perfect.
So, does this sample contain helium, or is it something else entirely? The answer often lies in the complexity of mixtures. Unknown gases frequently behave like “airport jungle juice” — a chaotic blend of elements emitting overlapping spectra that can confuse even experienced eyes.
This analogy isn’t just playful — it reflects a scientific challenge. When multiple elements coexist, their emission lines blend, making it tricky to nail down each one without robust analysis.
Why Detailed Analysis Matters
Simply seeing lines that resemble helium doesn’t guarantee helium’s presence. Scientists must examine the entire spectrum: checking which lines are missing, which extra ones pop up, and cross-referencing with known atomic data.
Advanced instruments and computer models help deconvolute these complex spectra, teasing apart contributions from different elements.
It’s like listening to an orchestra and trying to pick out each instrument’s unique sound in a noisy room. A careful ear — or in this case, a meticulous eye — is essential.
Practical Takeaways and Tips for Spectrum Enthusiasts
- Don’t jump to conclusions: An initial match between unknown and known spectra might be only superficial.
- Observe carefully: Focus on subtle differences — missing or extra emission lines matter a lot.
- Recognize mixtures: Complex spectra often indicate multiple elements are present, not just one “usual suspect.”
- Use reference data: Compare your emission lines with well-established spectra databases.
- Employ technology: Spectrometers and software improve identification accuracy drastically.
A Real-World Example
Think about how helium was discovered. Scientists observed bright yellow lines in the solar spectrum that didn’t match any known element on Earth at the time. This mystery light belonged to a new element only found in the Sun! Without emission spectra, helium might have remained hidden forever.
Now, imagine the excitement (and confusion) when spotting spectra in unknown gases that partially resemble helium’s lines. It’s a cosmic whodunnit requiring patience and sharp analysis, plus a dash of humor about “airport jungle juice.”
Final Thoughts: Why Emission Spectra Matter
The emission spectrum is more than just pretty lines on a chart; it’s a fundamental tool for understanding our universe. It lets us peek into elemental compositions of stars, planets, and mysterious gases. Yet, like any good detective story, there are twists. Sometimes light plays tricks, mixtures confuse the issue, and in these cases, careful examination makes all the difference.
Next time you glance at a starry sky, remember that a vibrant, invisible barcode is stretched across the cosmos. Scientists decode it to tell us who’s made of what, revealing secrets billions of years old — all from mere light.
So, are you ready to spot the “hidden helium” or unravel other spectral mysteries? Emission spectra challenge our perception and reward our curiosity. And that’s a bright idea worth sharing.
What is the emission spectrum used for in astronomy?
The emission spectrum helps identify elements in celestial bodies. Scientists analyze the light they emit to determine their chemical makeup.
Why can the emission spectrum of an unknown gas look similar to helium?
Some spectral lines may match at first glance, but further inspection shows differences. This suggests the unknown gas may not be pure helium.
How do mixtures affect the emission spectrum analysis?
Mixtures produce overlapping lines, making it hard to separate signals. Careful analysis is necessary to detect all components.
Can the emission spectrum confirm the exact elements in an unknown sample?
It can indicate which elements are likely present. But complex samples often require detailed study to avoid misidentifying elements.



Leave a Comment