Formula for Maxwell-Boltzmann Distribution Curve

The Maxwell-Boltzmann distribution curve is mathematically expressed by the formula:
f(v) = 4π (m / 2πkT)^(3/2) v2 exp(-mv2 / 2kT)
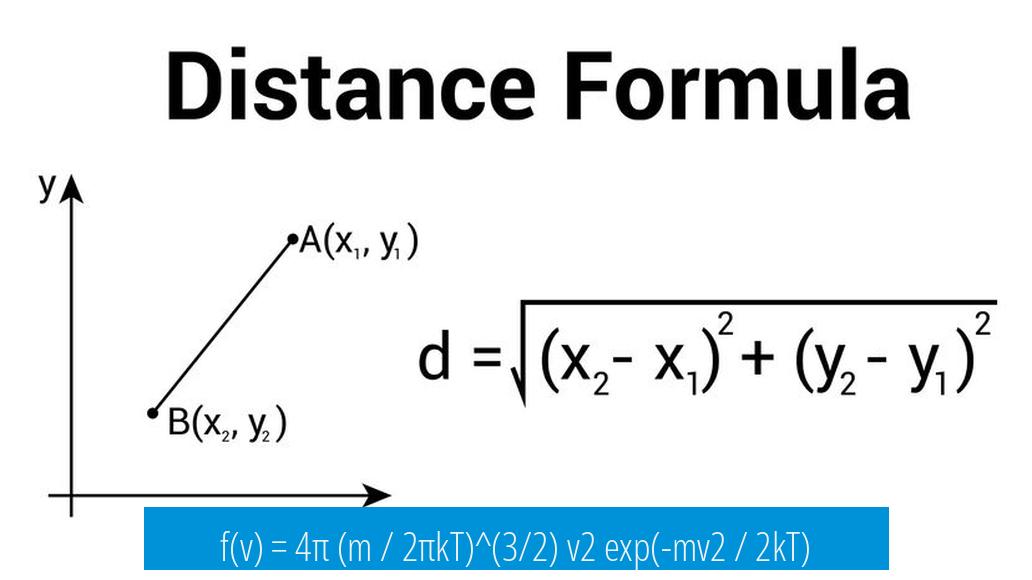
This formula represents the probability distribution function of molecular speeds in an ideal gas. Here, f(v) is the probability density of molecules moving with speed v, m is the mass of a gas particle, k is Boltzmann’s constant, and T is the absolute temperature.
Understanding the Derivation

Grasping the Maxwell-Boltzmann formula requires studying its derivation. The final equation forms through approximations and substitutions. It emerges from statistical mechanics principles, using kinetic theory of gases and the distribution of molecular energies.
A critical step is replacing the sum over discrete energy states with an integral over molecular velocities. This approach smooths out the discrete states into a continuous velocity spectrum.

Key Substitution in Derivation
- Define kinetic energy as \(E = \frac{1}{2}mv^2\).
- Replace summation by integration over velocity space.
- Incorporate exponential weighting by the Boltzmann factor \(\exp(-E/kT)\).
This procedure leads directly to the Maxwell-Boltzmann speed distribution formula noted above.

Locating the Distribution Function and Further Resources
The explicit distribution function is available in many standard references, including the Wikipedia page on the Maxwell-Boltzmann distribution.
For a rigorous and concise derivation, one can consult the open-source primer “The Necessity of Entropy: The Macroscopic Argument, the Microscopic Response and Some Practical Consequences” available at https://doi.org/10.48617/1029. This resource dedicates significant discussion to the microscopic foundations of thermodynamics and includes detailed steps for the derivation of the Maxwell-Boltzmann distribution.
Summary of Key Points
- Maxwell-Boltzmann formula expresses molecular speed distribution in gases.
- The formula is: \(f(v) = 4\pi \left(\frac{m}{2\pi kT}\right)^{3/2} v^2 e^{-mv^2/(2kT)}\).
- Derivation involves substituting kinetic energy into Boltzmann exponential factors and integrating over velocities.
- Refer to open-source thermodynamics primers for detailed mathematical development.
- The distribution function is widely available in scientific literature and online resources.
Formula for Maxwell-Boltzmann Distribution Curve: Unlocking the Velocity Secrets of Molecules
So, what exactly is the formula for the Maxwell-Boltzmann distribution curve? At its core, this famous curve describes how the speeds of molecules in a gas spread out at a given temperature — and the formula captures this beautifully:
f(v) = 4π (m / 2πkT)3/2 v2 e-mv2 / 2kT
Here’s the breakdown: f(v) represents the probability density of molecules moving at speed v. The constants include the molecule’s mass m, Boltzmann’s constant k, and temperature T. It’s not just a pretty formula— it reveals that molecular speeds are spread unevenly, clustering around a mode but tailing off for much slower or faster particles.
Why Should You Care About This Formula? The Story Behind the Math
It might seem intimidating at first glance. But trust me, diving into the derivation gives you a treasure chest of insight. Understanding where this formula comes from explains why molecules don’t all zip around at the same speed. It captures the dance of countless particles bouncing and colliding, each obeying classical mechanics but collectively creating a statistical distribution from chaos.
Most people just memorize the formula, but those who dig into its origin get to appreciate the subtle approximations and physics that make it tick. The truth: this curve arises by connecting kinetic energy and velocity distributions through probability and thermodynamics. It’s a brilliant intersection of physics and math.
Don’t Just Trust Me — Here’s Where to Find a Reliable Derivation
If you really want to geek out on the Maxwell-Boltzmann distribution, check out the open-source primer The Necessity of Entropy: The Macroscopic Argument, the Microscopic Response and Some Practical Consequences. This resource lays out the foundations of thermodynamics with clarity and rigor.
In the “micro” section of this concise work (17 pages plus appendices), you’ll find step-by-step derivation from first principles. It’s not just dry theory — you get to see the role entropy plays and why integrals replace sums over discrete states to handle continuous velocity space.
An Essential Hint for the Derivation: The Magic Substitution
Don’t get lost in the math maze. When tackling the derivation yourself, pay attention to a key substitution in equation (34) of the primer: Replace the kinetic energy E with E = 1⁄2 mv2. This ties particle energy directly to velocity, which lets you switch from summing over energy states to integrating over velocity values.
It sounds technical, but this move is crucial. It transforms your problem from dealing with discrete quantities to embracing continuous velocity distributions, making the Maxwell-Boltzmann formula emerge naturally.
How Does This Play Out in Real Life? Benefits and Uses of the Maxwell-Boltzmann Curve
The Maxwell-Boltzmann distribution isn’t just textbook trivia. Its applications ripple through chemistry, physics, and engineering. For instance, predicting reaction rates hinges on knowing how many molecules surpass a threshold speed or energy. The curve informs how fast gases diffuse, how rockets manage exhaust velocity, and even atmospheric science.
Engineers designing combustion engines rely on it to model fuel particle collisions. Meanwhile, astrophysicists study star atmospheres applying these principles to gas motions at extreme temperatures. The ramifications stretch from microscopic molecules to cosmic scales!
Personal Experience: Why I Recommend Learning the Derivation
Back when I first met the Maxwell-Boltzmann distribution, it felt daunting—just a scary exponential blob. Then I unraveled how the kinetic energy substitution worked, and the veil lifted. Suddenly, the curve made intuitive sense. Speed is tied to energy, and their statistical interplay shapes molecular behavior.
This clarity changed my perspective. Greater insight came from understanding the derivation, not just memorizing the formula. I encourage anyone curious about physics to spend time with the steps. It’s rewarding, and the result is a versatile tool you can apply across science and engineering.
Want to Try It Yourself? Here’s a Quick Walkthrough
- Start from the idea that molecules have discrete energy states. Sum over these energy states.
- Use Boltzmann’s factor, e-E/kT, to weight states by their energy probability.
- Substitute kinetic energy E = 1⁄2 mv2 to connect energy and velocity.
- Replace the sum with an integral over velocity space, since molecular speeds vary continuously.
- Incorporate normalization factors to ensure the total probability sums to one.
- Derive the function f(v) as above, complete with the v2 term that shapes the curve.
Before you know it, you obtain the Maxwell-Boltzmann formula—no mysterious black box, just a logical progression.
How Does the Maxwell-Boltzmann Formula Stack Up Against Other Distributions?
Great question! Unlike uniform or normal distributions, the Maxwell-Boltzmann distribution is skewed right with a distinct peak. It reflects the physical reality that molecules rarely stand still or reach extreme speeds. Instead, most bunch up near a moderate speed, with fewer molecules at very low or very high velocities.
This contrasts sharply with Bose-Einstein or Fermi-Dirac distributions, which apply quantum statistics. Maxwell-Boltzmann reigns supreme in classical ideal gases where quantum effects can be ignored. So, knowing which distribution to use offers practical guidance for scientists.
In Conclusion: The Maxwell-Boltzmann Distribution Formula Is More Than Math — It’s a Window Into Molecular Motion
From our lively discussion, it’s clear the Maxwell-Boltzmann distribution curve stands as a cornerstone in statistical mechanics. The formula encapsulates an incredible story: particles in motion, guided by energy and temperature, collectively forming a beautiful pattern.
If you only remember one fact, let it be this all-important formula:
f(v) = 4π (m / 2πkT)3/2 v2 e-mv2 / 2kT
Look deeper—learn the derivation steps, especially the kinetic energy substitution and integral transition. Then watch as abstract symbols become an accessible portrait of molecular speeds.
Curious to explore further? Grab the primer linked above and experience the thrill of theory meeting reality. You’ll join a lineage of scientists who uncovered one of nature’s fundamental laws with clarity and elegance. Time to let those molecules reveal their speed secrets to you!
What is the formula for the Maxwell-Boltzmann distribution curve?
The formula describes the probability distribution of particle speeds in a gas. It involves an exponential term with kinetic energy and a term for velocity squared. It models how particles spread over speeds in thermal equilibrium.
Where can I find the Maxwell-Boltzmann distribution function?
The distribution function can be found on Wikipedia. It provides a clear expression and explanation of the formula along with its variables and usage.
How is the Maxwell-Boltzmann distribution formula derived?
Deriving the formula uses statistical mechanics and thermodynamics. It requires substituting kinetic energy as (1/2)mv² and turning sums over discrete states into integrals over velocity ranges.
Is there a detailed resource to understand the derivation of the Maxwell-Boltzmann distribution?
Yes. An open-source primer titled “The Necessity of Entropy” covers this. It presents the microscopic foundations of thermodynamics and explains the derivation in detail, accessible online.
What is a key substitution step in the Maxwell-Boltzmann distribution derivation?
One key step is replacing energy E by kinetic energy E = (1/2)mv². Another is switching from summation over states to integration over velocity values to handle continuous speed distributions.




Leave a Comment