Freezing Point of Sulfuric Acid
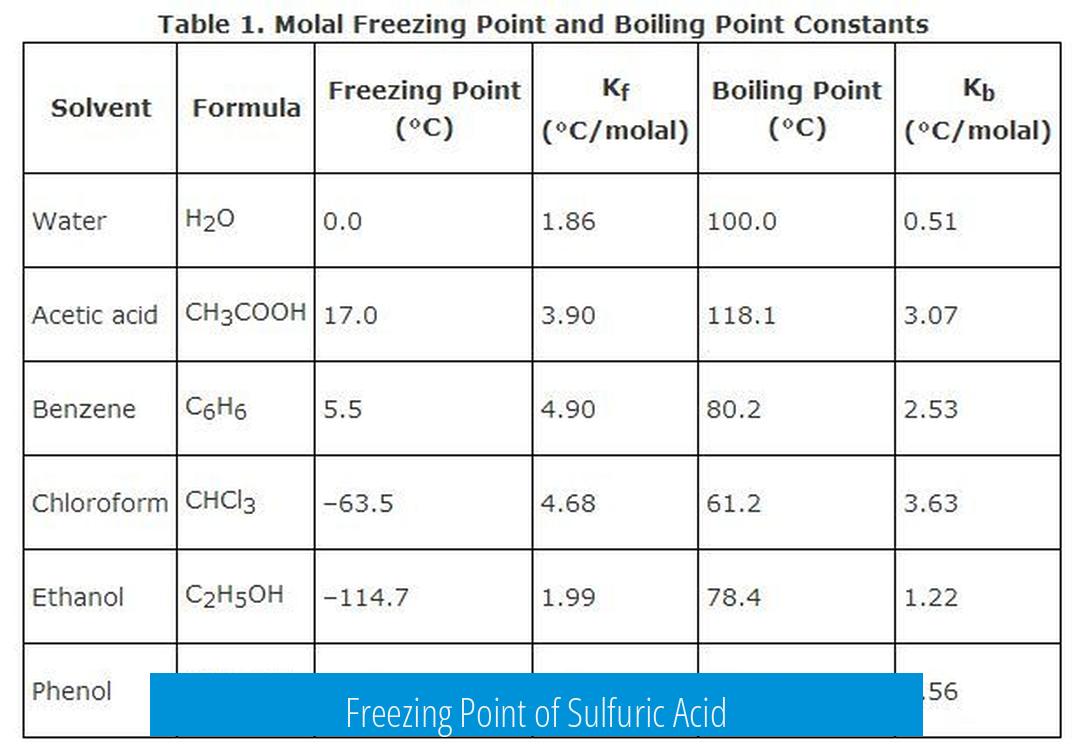
The freezing point of pure sulfuric acid (H2SO4) is approximately 10.3 °C (50.5 °F), but this value varies with concentration due to its complex phase behavior and crystallization properties. Sulfuric acid’s freezing characteristics differ significantly from those of simple solvents because of its strong acidity and hydrogen bonding.
Phase Transitions and Solidification
When sulfuric acid freezes, it undergoes distinct physical transitions manifesting as discontinuities, relating to changes in molecular arrangement and crystallization. These transitions can be identified by careful thermal analysis, although labeling each transition precisely remains challenging due to complex interactions at the molecular level.
Solidification causes the acid to expand, a notable peculiarity because most liquids contract upon freezing. This expansion reflects the rearrangement of H2SO4 molecules and the formation of a crystalline lattice that occupies more space.
Properties of Solid Sulfuric Acid
In its frozen state, sulfuric acid maintains its chemical integrity, but its reactivity is altered. The solid retains acidic properties, although the mobility of ions and molecules is reduced. This reduced mobility slows reactions but does not eliminate the acid strength.
Practical Applications of Frozen H2SO4
Frozen sulfuric acid finds specialized use in chemical synthesis. For instance, adding solid reagents to frozen H2SO4 allows controlled reactivity as the acid melts. This method serves as an alternative to gradual dropwise addition, enabling safer and more precise reaction management.
Challenges in Modeling Solid-Liquid Transitions
Accurately modeling the solid-liquid behavior of sulfuric acid remains difficult. The substance exhibits complex hydrogen bonding both within molecules (intramolecular) and between molecules (intermolecular), complicating prediction of crystal geometry and interactions.
- Hydrogen bond orientation varies with temperature and concentration.
- Conformational flexibility of molecules adds to modeling challenges.
- The term “specific interactions” is used due to incomplete theoretical understanding.
Crystallization and Composition Variations
Sulfuric acid’s crystallization is sensitive to concentration. At around 94% concentration and low temperatures (~–30 °C), agitation can produce mixtures of crystals with slightly different acid concentrations (e.g., 93% and 95%). This phenomenon highlights complex phase equilibria that influence solid formation.
Key Takeaways
- Pure sulfuric acid freezes near 10.3 °C; freezing point varies with concentration.
- The acid expands upon freezing, unlike many liquids.
- Frozen sulfuric acid retains acidic properties but reacts more slowly.
- Frozen acid provides a controlled medium for some chemical syntheses.
- Modeling solid-liquid transitions is complex due to hydrogen bonding and molecular flexibility.
- Crystallization can yield crystals with varying acid concentrations depending on conditions.





Leave a Comment