Understanding HFIP: Properties, Hazards, and Safe Handling

HFIP (Hexafluoroisopropanol) is a toxic, volatile fluorinated solvent with distinct irritant properties and a strong unpleasant odor. Despite its reputation, it is less hazardous than many related compounds but demands careful handling.
What is HFIP?
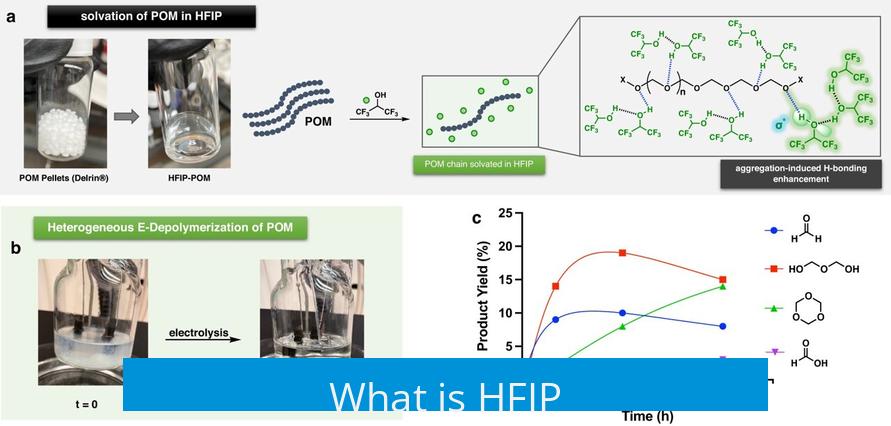
HFIP stands for hexafluoroisopropanol. It is a fluorinated alcohol, widely used in chemical synthesis and research. This compound is a low-boiling-point liquid, highly volatile, with distinct solvent properties.
Its unique chemical structure, bearing six fluorine atoms attached to an isopropanol backbone, grants HFIP strong polarity and hydrogen bonding capability. These features make it valuable as a solvent in peptide synthesis, polymer science, and as a reagent in organic chemistry.
Toxicity and Irritant Effects
HFIP is toxic and a strong irritant. Its toxicity arises from both skin contact and inhalation. While less corrosive than trifluoroacetic acid (TFA), HFIP can still irritate skin, eyes, and mucous membranes.
On a relative hazard scale from 1 to 10 (with 10 being the most hazardous), HFIP rates around 2 or 3. This means it carries moderate risk when handled improperly but is not among the most dangerous lab chemicals.
Comparison with Related Fluorinated Compounds
- Trifluoroethanol (TFE): Related compound and considered more hazardous, particularly as a reproductive hazard.
- Trifluoroacetic Acid (TFA): More corrosive but similar in handling precautions.
- Halogenated solvents (Chloroform, Dichloromethane): More toxic and known carcinogens, placing them above HFIP in terms of risk.
Despite the general concern about fluorinated chemicals, HFIP’s toxicity is often overestimated. This stems from confusion with more hazardous counterparts, like trifluoroethanol, which presents higher risks.
Exposure Risks and Odor Characteristics
HFIP emits a very strong, unpleasant odor often described as similar to cat urine. This odor serves as a warning sign during laboratory use.
Inhalation of HFIP vapors is a primary concern. The compound’s volatility tends to release enough fumes for noticeable odorous exposure quickly. Users must always operate HFIP inside well-ventilated fume hoods to avoid inhaling vapors.
Direct inhalation can cause irritation of the respiratory tract and discomfort. The potency of the odor also signals the presence of potential exposure, warranting immediate action to minimize contact.
Recommended Protective Measures
When working with HFIP, the following safety procedures are essential:
- Use a fume hood: Prevents inhalation of vapors and contains the strong odor.
- Wear gloves: Nitrile gloves provide some resistance but may not fully prevent permeation of HFIP. Consider double gloving or more resistant glove materials.
- Eye protection: Goggles or face shields protect the eyes from splashes or accidental contact.
- Protective clothing: Lab coats and long sleeves minimize skin exposure.
Spilling HFIP on the skin can cause irritation and more severe effects due to its volatility. Use caution when dispensing or transferring this solvent.
HFIP handling protocols closely resemble those for TFA, though HFIP is less corrosive. Following proper procedures reduces nearly all risks associated with this solvent.
How HFIP Compares to Other Solvents
| Aspect | HFIP | Trifluoroethanol (TFE) | Chloroform/Dichloromethane (CHCl3/DCM) |
|---|---|---|---|
| Toxicity | Moderate (rating 2-3/10) | Higher; reproductive hazard | High; known carcinogens |
| Irritancy | Strong irritant, less corrosive than TFA | Strong irritant, more hazardous on skin | Irritant plus carcinogenic risks |
| Boiling Point | Low; volatile | Low; volatile | Moderate to low; volatile |
| Handling Requirements | Fume hood, gloves, eye protection | More stringent due to higher hazard | Strict; regulated use |
HFIP stands out as a fluorinated compound with lower hazard, particularly when compared to halogenated solvents like chloroform or dichloromethane, both listed carcinogens. These latter solvents require more rigorous lab controls and exposure monitoring.
Addressing the Negative Reputation of Fluorinated Solvents
Fluorinated chemicals often carry a bad reputation. This is associated with their environmental persistence, bioaccumulation potential, and toxicity seen in some compounds.
However, not all fluorinated solvents share these concerns equally. HFIP has a specific toxicity profile that is less severe than some analogs but must still be respected for its irritant nature and volatility.
Misunderstanding and overgeneralization fuel the negative perception. Scientists handling HFIP report that with proper safeguards it is manageable and does not pose excessive risks.
Summary of Safe HFIP Use in Laboratories
- HFIP is a low-boiling fluorinated solvent with strong irritant properties and a pungent odor.
- It poses moderate toxicity risk; less hazardous than trifluoroethanol or halogenated carcinogens.
- Handling precautions include use in fume hoods, wearing gloves, eye protection, and minimizing skin contact.
- The unpleasant odor serves as a safety indicator to avoid exposure.
- Despite negative perceptions, HFIP’s hazard is manageable with correct laboratory practices.
Additional Considerations
Laboratories using HFIP are encouraged to maintain up-to-date safety data sheets (SDS) and adhere to institutional safety protocols. Spill kits and proper waste disposal means should be in place due to HFIP’s volatility and toxicity.
Future research and industrial applications may further clarify HFIP’s environmental impact, though current focus is on safe handling and minimizing direct human exposure.
Key Takeaways
- HFIP is a toxic, volatile, fluorinated solvent requiring careful handling.
- It is a moderate hazard, less severe than related fluorinated alcohols and halogenated carcinogens.
- Use fume hoods and personal protective equipment when working with HFIP.
- Its strong, unpleasant odor warns of potential exposure through inhalation.
- Negative reputations stem mostly from association with similar but more hazardous compounds.
HFIP? Not a Lot of Info Online and Fluoridated Stuff’s Bad Rep – What Do You Really Know?
So, what is HFIP, and is the fuss about it really justified? Simply put, HFIP (Hexafluoroisopropanol) is a toxic, low-boiling, fluorinated solvent that smells horrendously like cat pee but is less hazardous than many related fluorinated compounds. Despite its reputation, it’s manageable if you treat it with respect.
Now let’s dive deeper and clear the fog surrounding this not-so-common yet important chemical that often gets lumped unfairly into the “toxic fluoridated stuff” category.
Understanding HFIP’s Toxicity – It’s Not as Scary as You Think
First off, HFIP is toxic and a strong irritant. Imagine it as a substance that can irritate your skin and respiratory tract, but thankfully it doesn’t score a perfect 10 on the danger scale. Experts rank it around 2 or 3 out of 10 — far from the most hazardous. That’s still enough reason to be cautious but no need to panic.
“Treat it like trifluoroacetic acid (TFA) at the very least — gloves on, and definitely under a fume hood.”
Why the confusion then? Well, many people mix up HFIP with trifluoroethanol, its close chemical cousin. The latter is a recognized reproductive hazard and probably worse when it touches your skin. So, if fear of trifluoroethanol spills over into HFIP awareness, that’s where the misconceptions arise.
HFIP’s toxicity seems exaggerated due to these associations. In reality, if you follow standard lab safety procedures, your risk is pretty modest.
That Smell! Should You Stay Away Because of the Odor?
Let’s talk about what you’ll notice even before handling the chemical — its odor. People who have worked with HFIP often describe it as smelling like cat pee. Yes, it’s as bizarre as it sounds.
This smell is not just unpleasant; it’s a sign to keep your distance or better yet, use a fume hood.
- Inhalation is a primary risk here.
- Don’t chance breathing it in where ventilation is poor.
- That pungent scent feels like “being punched in the face” to some chemists!
One could say the smell itself is a built-in warning system to respect this chemical’s power.
Protective Gear — How to Handle HFIP Safe and Smart
Gloves? Absolutely. But a heads-up: nitrile gloves may not provide full protection because halogenated solvents can seep through some glove materials or evaporate around the edges.
Work inside a fume hood, always. The hood caps off exposure risks by ventilating fumes and containing spills. If you’ve ever spilled HFIP on your skin due to glove failure, you’d know why extra caution pays off.
Compared to trifluoroacetic acid, HFIP is less corrosive but still demands similar lab discipline.
How HFIP Measures Up to Other Halogenated Solvents
Ever heard of dichloromethane (DCM) or chloroform? Those two are notorious — they’re known carcinogens, appearing on official carcinogen lists. Their hazard level dwarfs HFIP’s.
If you’ve handled DCM or chloroform, you know the drill:
- Maximal ventilation.
- Strict protective gear.
- Minimizing exposure time.
HFIP doesn’t make that cut for carcinogenic warnings. So how come it gets some bad rep?
Mostly because anything fluorinated often scares people. Fluoride hangs out in your toothpaste and water, and while it has benefits, it also has folks worried. The toxicity of other fluorinated solvents contributes to HFIP’s reputation, even if that’s not entirely fair.
Why Use HFIP at All, Then?
In research and industrial settings, HFIP has unique value. It can dissolve difficult compounds and help tweak properties for novel materials. Its fluorinated nature gives it special properties hard to replace with ordinary solvents.
Given its utility and moderate hazard, those who handle HFIP daily know how to minimize risks.
Key Takeaway: Be Smart, Not Scared
| Property | HFIP | Trifluoroethanol | Dichloromethane (DCM) |
|---|---|---|---|
| Toxicity Level (scale 1-10) | 2 to 3 | Higher; reproductive hazard | High; carcinogen |
| Odor | Strong, cat pee-like | Less unpleasant | Chloroform-like sweet smell |
| Corrosiveness | Less than TFA | Moderate | Moderate |
| Protective Equipment | Gloves + fume hood | Gloves + fume hood | Rigorous PPE + ventilation |
| Carcinogenicity | No clear warning | No clear warning | Known carcinogen |
Practical Tips When Handling HFIP
- Always use a fume hood to avoid inhaling fumes.
- Wear gloves, but be mindful—nitrile gloves may not be fully protective.
- Keep the container sealed when not in use to control odor and vapor release.
- Store away from incompatible chemicals.
- If a spill happens, clean it immediately with appropriate absorbents and ventilate well.
Final Words
HFIP’s reputation as “scary toxic fluoridated stuff” is a bit overblown. Its odor is quite memorable, but with proper precautions, its risks are manageable—less than those from many other halogenated solvents. With gloves, a fume hood, and a bit of respect for this funky smelling chemical, you can safely use HFIP in your lab or project.
So next time someone tells you “stay away from fluorinated solvents,” you can say, “I know HFIP’s quirks – it smells bad, sure, but it’s really not the villain everyone thinks it is!”
What is HFIP and how toxic is it?
HFIP is a fluorinated solvent that is toxic and strongly irritant. It is less hazardous than trifluoroethanol but still requires careful handling. Its toxicity level rates about 2 or 3 out of 10, where 10 is most dangerous.
Why does HFIP have such a bad reputation?
HFIP’s bad reputation mostly comes from confusion with more toxic fluorinated compounds like trifluoroethanol. While it is toxic, the risk is often overestimated and manageable with proper lab safety.
How should I handle HFIP safely in the lab?
Use HFIP in a fume hood due to its irritating vapors and unpleasant odor. Wear gloves, but be aware nitrile gloves may provide limited protection against skin contact. Avoid inhaling its fumes.
What does HFIP smell like and why does that matter?
It has a strong odor often compared to cat urine. This smell alerts you to exposure, so using a fume hood is essential to avoid inhalation and discomfort.
How does HFIP compare to other solvents like chloroform or dichloromethane?
HFIP is less hazardous than carcinogenic solvents such as chloroform and dichloromethane. While still toxic, HFIP’s risks are lower when proper precautions are followed.



Leave a Comment