How Did Mendeleev Arrange the Elements in the Periodic Table in 1869 Without Knowledge of Electrons, Protons, or Neutrons?
Dmitri Mendeleev successfully arranged the elements in the periodic table in 1869 by organizing them according to atomic masses and recurring chemical properties, despite lacking knowledge of subatomic particles such as electrons, protons, and neutrons. He used patterns in element masses and chemical behavior to build a periodic classification that predicted missing elements and their properties. His approach combined systematic observation, classification, and logical deduction from known data.
1. Arrangement Based on Atomic Mass and Chemical Properties

At the time of Mendeleev’s work, the concept of atomic number or subatomic structure did not exist. However, chemists could determine the atomic masses of elements through experimental methods. Mendeleev capitalized on this information.
Use of Atomic Mass
- Mendeleev arranged known elements roughly in order of increasing atomic mass.
- Atomic mass was experimentally measurable, unlike atomic number, which was unknown.
- Arranging elements by weight inadvertently reflected increasing proton count, which would be understood decades later.
Grouping by Similar Properties
Mendeleev grouped elements vertically in families based on similarities in chemical properties such as reactivity, valence, and oxide formulas. This grouping demonstrated repeating periodic trends.
- He observed that elements with similar chemistry appeared at regular intervals when arranged by atomic mass.
- He created cards for each element detailing mass and observable chemical behavior to physically rearrange and test patterns.
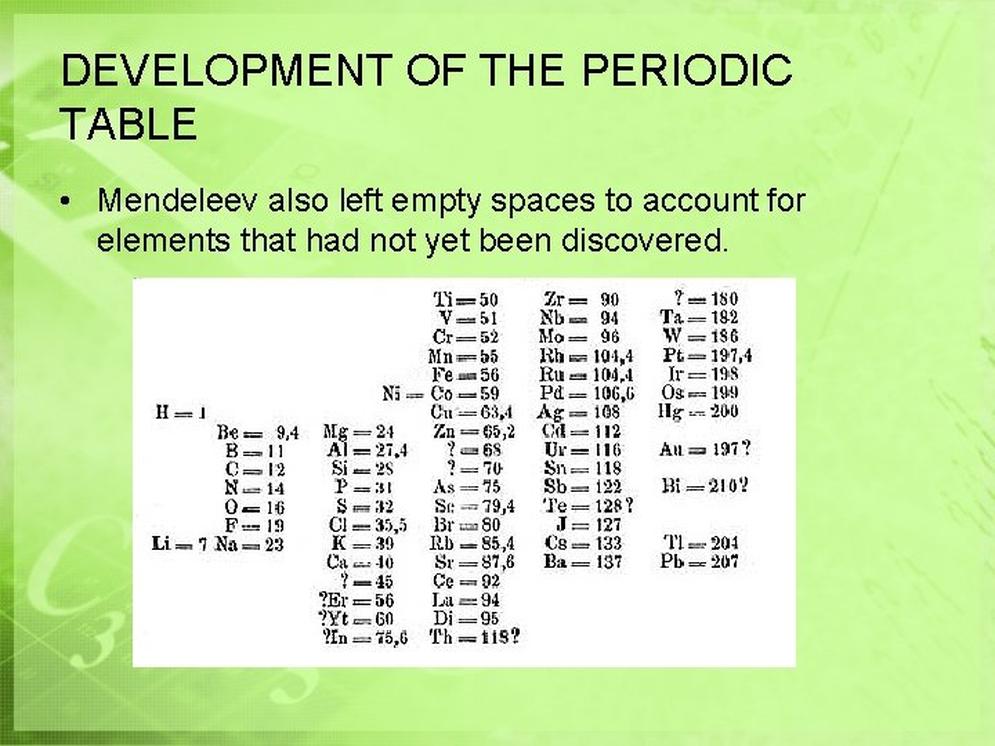
- Leaving gaps in the table marked positions for undiscovered elements, whose existence and properties could be predicted.
Recognition of Periodic Patterns
He noticed that every eighth element shared similar traits, an early form of the periodic law known as the “law of octaves.” The repeating groups of properties became more apparent with time and analysis.
2. Predictive Power: Gaps and Undiscovered Elements
A significant strength of Mendeleev’s table was its ability to predict elements not yet discovered.
Predicting Missing Elements
- Mendeleev purposely left gaps where no known element fit the periodic pattern.
- He estimated the masses and chemical properties of these missing elements based on trends.
Example: Germanium
When germanium was isolated, its properties matched Mendeleev’s predictions for the element he called “eka-silicon.” Even before seeing germanium, Mendeleev corrected the reported density of the new element, demonstrating his predictive approach’s accuracy (~245 characters).
Example: Eka-Aluminium (Gallium)
Mendeleev predicted another element, eka-aluminium, later discovered as gallium. Its properties perfectly matched his forecast, reinforcing the validity of his classification system.
3. Methodology and Prior Influences
Data Used
- Chemical formulas: Oxides, chlorides, hydrides.
- Atomic weights obtained from experiments available at the time.
- Molar volumes and physical properties like density.
- Descriptive characteristics, such as reactivity and valence behavior.
Influence of Earlier Work
Mendeleev built on chemical trends identified by predecessors, such as Döbereiner’s triads, grouping elements in threes with average properties. He incorporated Dalton’s atomic weight measurements and noticed recurring patterns in elemental behavior, setting the stage for his periodic arrangement.
Trial, Error, and Revision
The table was not a sudden revelation but evolved through extensive trial and error. Mendeleev shuffled element cards repeatedly to find the best overall fit. During this process, he recognized anomalies and corrected misplaced elements by emphasizing chemical similarity over strict atomic mass order.
Example of Reordering
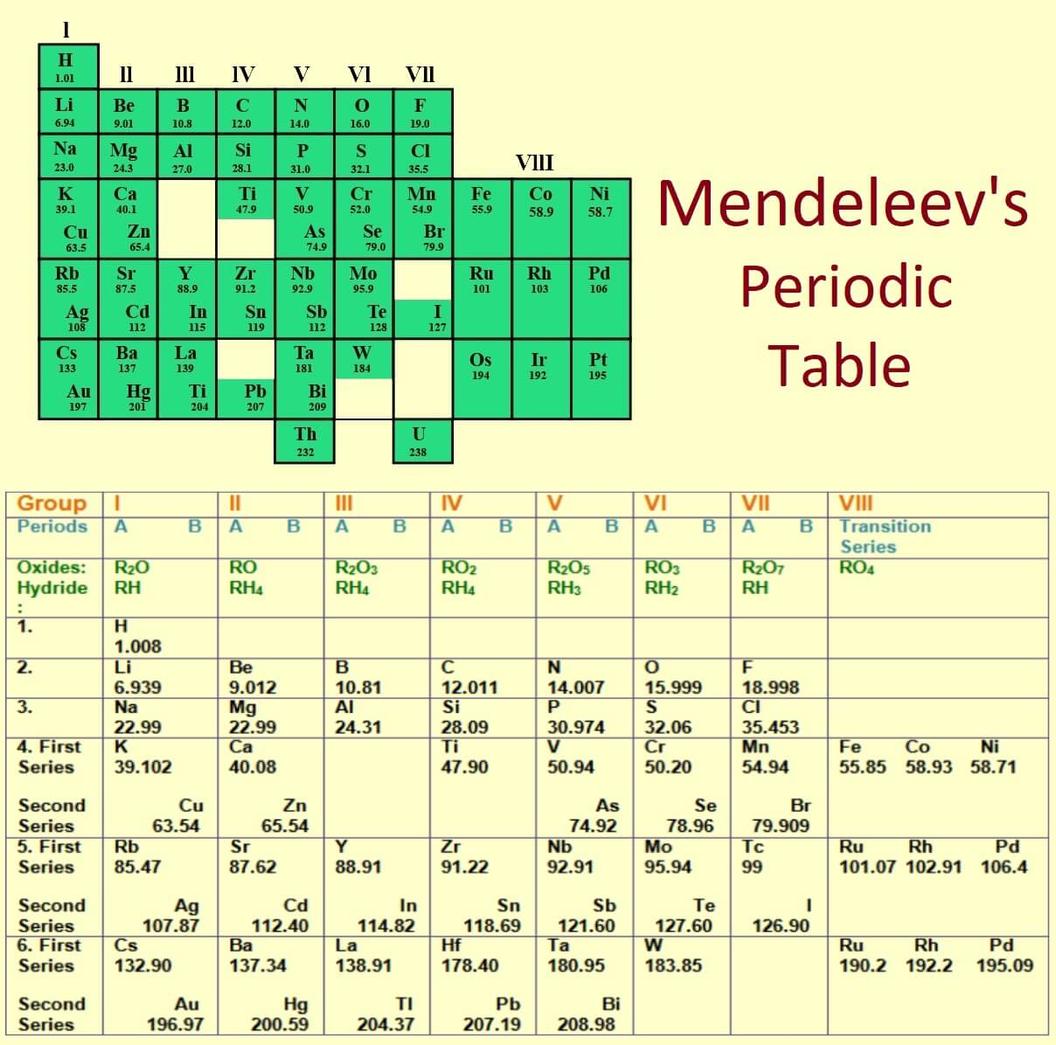
He placed tellurium ahead of iodine despite tellurium having a higher atomic mass. His chemical evidence indicated tellurium’s properties aligned with selenium and iodine’s with bromine. He attributed the reversed mass order to measurement errors available at that time.
4. Addressing Lack of Atomic Number and Subatomic Knowledge
Unknown Atomic Numbers
Atomic number—the actual number of protons determining an element’s identity—was discovered decades later. Mendeleev lacked this information entirely.
Atomic Mass as a Proxy
He used atomic mass as the primary ordering parameter, reasoning it generally increased with element number.
Some inconsistencies arose, but he corrected them by prioritizing chemical properties and similarities.
Recognition of Measurement Errors

Mendeleev accepted that some atomic masses were inaccurately measured and adjusted his table accordingly, boldly predicting the true properties and masses of elements before experimental verification.
5. Conceptual Insights: Discovering Patterns Without Full Atomic Theory
Mendeleev’s achievement exemplifies how scientific insight can precede theoretical understanding.
Discovering Patterns Before Explanation
- He identified periodic trends before knowledge of electron configurations or nuclear structure.
- The periodic table anticipated atomic numbers and fundamental atomic structure.
- It is common in science to discover patterns first then seek explanations, a process still observed in many fields.
Parallel in Chemical Bonding
Similar to the periodic table, the octet rule and bonding theories were formulated before underlying quantum mechanics explained them decades later.
6. Collaborative and Historical Context
Mendeleev was not alone. Several chemists independently explored elemental classification. His version surpassed others due to predictive accuracy and systematic methodology.
Rivals and Evolution
- Contemporaries such as Lothar Meyer developed early periodic tables with similar ideas.
- Constant refinement and experimental testing shaped the final table’s form.
Additional Experimental Approaches
Burning pure elements and measuring oxygen absorption helped determine oxidation states and validate group placements.
Mendeleev’s Method Explained in His Own Words
Mendeleev compared his method to arranging a deck of cards with known properties but no numbers, continually reorganizing them to reveal patterns and noting that gaps improved the system.
This analogy illustrates his practical, hands-on approach to classification that relied on observable chemical data rather than unknown atomic structures.
Summary of How Mendeleev Managed to Develop the Periodic Table Without Knowledge of Subatomic Particles
- Ordered elements primarily by atomic mass, a known physical quantity.
- Grouped elements vertically by similarity in chemical properties, such as reactivity and compound formulas.
- Identified periodicity and repeating patterns in elemental characteristics.
- Inserted gaps for undiscovered elements and predicted their properties accurately.
- Prioritized chemical behavior over strict atomic mass when inconsistencies appeared.
- Relied on trial, error, and chemical insight, building on earlier classifications like Döbereiner’s triads.
- Validated his predictions with later experimental discoveries (e.g., gallium and germanium).
His work preceded and anticipated atomic number and electronic structure, marking a landmark in the history of chemistry.
How did Mendeleev manage to arrange the elements in a periodic table in 1869, given that he had no knowledge of electrons, protons, or neutrons?
Simply put, Mendeleev arranged the elements by their increasing atomic masses combined with their chemical properties, revealing a repeating pattern without knowing about subatomic particles at all.
Sounds like magic? Well, it’s actually a brilliant example of observational science mixed with clever detective work. He didn’t have electrons, protons, or neutrons in his toolbox. Instead, he wielded atomic masses and chemical behaviors as his clues. Let’s dive into how this periodic table wizard pulled it off.
Playing Cards with Elements: Mendeleev’s Hands-On Method
Imagine a kid with a deck of Pokémon cards, except these cards are the known chemical elements in the 1860s. Mendeleev literally wrote each element’s properties—mass, oxide formulas, reactivity—on cards and spread them out on the table.
Then, he shuffled and rearranged them, grouping those that seemed to share similar traits and lining them up by increasing atomic mass. Through this hands-on trial-and-error, he noticed some spots where no suitable card existed. Instead of forcing things, he cleverly left gaps. This is how he hinted that unknown elements were yet to be discovered.
The Spark: Atomic Mass and Properties
How could mass alone be so powerful? Back then, atomic mass was the best scientists could calculate. Though atomic number—number of protons—was still a mystery, atomic mass mostly reflected this number since heavier elements generally have more protons.
Mendeleev noticed a pleasing pattern: If you line up elements by increasing atomic mass, their chemical properties repeat periodically. For example, alkali metals like lithium, sodium, and potassium shared reactivity and clustered in groups.
He combined this with formulas of oxides, chlorides, hydrides, and other measurable properties. Suddenly, the chaotic list of elements became a patterned map of the chemical world. This “periodicity” was Mendeleev’s big aha moment, revealing the first periodic law.
When Masses Don’t Tell the Full Story: Making Adjustments with Chemistry
Of course, atomic mass measurements weren’t perfect. Some were off, due to experimental errors or natural isotopes unknown at the time. Mendeleev spotted contradictions, especially with tellurium and iodine. Tellurium’s mass is higher, yet chemically it fit better before iodine.
Here came the genius: he trusted chemical behavior over atomic mass and swapped their order. He assumed the mass of tellurium or iodine was measured incorrectly—and later, atomic number would vindicate him by showing tellurium has more protons but a lower atomic mass due to isotopes. Talk about reading between the lines!
Predicting the Unknown: The Table’s Prophetic Power
Mendeleev went beyond labeling what was known. He boldly predicted missing elements, their atomic masses, and even their chemical properties. His predictions were astonishingly precise.
Take eka-aluminium, a placeholder name for one such predicted element. When gallium was discovered, it fit Mendeleev’s forecasts like a glove: similar properties, atomic mass close to his prediction.
Then came germanium, which had baffled scientists. When it was found, Mendeleev wrote to the discoverer to correct the measured density, even though he never laid eyes on the sample. Turns out he was right again. This uncanny foresight gave his table unparalleled credibility and helped cement the periodic table’s foundation.
Building on Giants and Collaborators
Mendeleev didn’t invent the idea from nothing. He stood on the shoulders of earlier scientists, such as Döbereiner, who identified triads—groups of three elements with related properties and average weights. These tiny patterns hinted at larger, yet undiscovered structures.
Many chemists were scratching their heads for years, but Mendeleev connected the dots better than anyone. He also welcomed revisions, knowing that science is seldom a straight path. His table evolved through trial, error, and debate. Sometimes he changed the ordering, introduced gaps, and adjusted as new data rolled in.
Why Did His Approach Work Without Knowing Atoms’ Inner Workings?
Isn’t it crazy that Mendeleev cracked the periodic code before anyone ever knew about protons or electrons? Chemistry is full of such mysteries where patterns come before understanding.
For instance, the octet rule—electrons sharing or transferring to fill an outer shell—was understood decades later with quantum mechanics. However, chemists correctly used it to explain bonding long before orbitals and subatomic particles were discovered.
Similarly, Mendeleev’s arrangement wasn’t arbitrary—it reflected a deep, natural order manifesting in observable properties and atomic masses. His intuition about the periodic repetition was sound, and later atomic theory gave it scientific backing.
Methodological Insights: The Making of the Table
Mendeleev’s toolbox was full of chemical formulas and physical properties. He worked with oxides, chlorides, hydrides, and examined their atomic weights and molar volumes—essential data in his era.
He tested his assumptions repeatedly, debating placement of elements and refining predictions. The occasional need to reorder elements (e.g., swapping iodine and tellurium) revealed his commitment to chemical evidence over numerical rigidity.
This methodical process shows that science often marches forward on patterns, predicates, and practical observations rather than perfect theory. Mendeleev was a master pattern seeker in a world without atomic theory.
Why Should We Care About Mendeleev’s Table Today?
Mendeleev’s periodic table revolutionized chemistry. It gave chemistry a structured language—no longer a chaotic list, but a coherent map of element behavior. It helped scientists predict elements and understand chemical relationships.
Even today, the periodic table serves as a fundamental resource for chemists, educators, and students worldwide. Mendeleev’s story reminds us that observation and pattern recognition can precede and even predict deep scientific knowledge. Fancy that—a real-life science detective!
Some Takeaways and Practical Lessons
- Trust the data, but not blindly: Mendeleev showed that when data conflicts with observation, it’s okay to question measurements.
- Leave gaps to accommodate the unknown: Scientific humility by recognizing what we don’t know can drive discovery.
- Utilize pattern recognition: Observing recurring themes leads to breakthroughs, even without full theoretical understanding.
Final Thoughts: The Periodic Table as a Triumph of Pattern over Knowledge
Mendeleev’s periodic table is the perfect example of seeing the forest, even if you don’t yet understand every tree. By organizing elements according to atomic mass and grouping by properties, he stumbled on a natural pattern underpinning the material world.
He didn’t need electrons, protons, or neutrons—all that quantum jiggle and nuclear complexity would come later. His periodic table predicted elements and set the stage for the atomic discoveries that followed. It’s a testament to keen observation and the power of scientific intuition.
Next time you glance at the periodic table on your classroom wall or your computer screen, remember—this chart was pieced together with a deck of cards, a heap of curiosity, and a dash of genius before anyone even dreamed of atoms being divisible.
References and Further Exploration:
- UCSD Library: Mendeleev’s First Tables
- Origins at OSU: Mendeleev and the Periodic Table
- Greenwood and Earnshaw’s Chemistry of the Elements
- Oliver Sacks’s Uncle Tungsten
How did Mendeleev arrange elements without knowing about protons or electrons?
Mendeleev arranged elements by atomic mass and chemical properties. He noticed patterns in how masses increased and grouped elements with similar traits vertically. This created a repeating pattern, even without knowledge of subatomic particles.
How did Mendeleev predict elements that were not yet discovered?
He left gaps in his table for unknown elements. By analyzing patterns in atomic masses and properties, he predicted their existence and characteristics. For example, he predicted eka-aluminium, later discovered as gallium.
Why did Mendeleev switch the order of some elements despite their atomic masses?
He prioritized chemical similarities over strict mass order. For instance, he placed tellurium before iodine because their properties matched better, assuming atomic mass measurements might be inaccurate for some elements.
What tools and ideas did Mendeleev use to build his table?
- Chemical formulas of oxides, chlorides, hydrides
- Atomic weights and molar volumes
- Prior work like Döbereiner’s triads
- Trial, error, and iterative rearrangement of element cards
How was Mendeleev’s periodic table a breakthrough despite lacking modern atomic theory?
His table revealed a clear pattern predicting properties and element order before atomic numbers were known. This pattern held true and led to the discovery of many elements fitting his predictions, paving the way for atomic theory development.





Leave a Comment