How Do We Exactly Know One Carbon Atom Has 6 Electrons or Oxygen Has 8 Electrons?
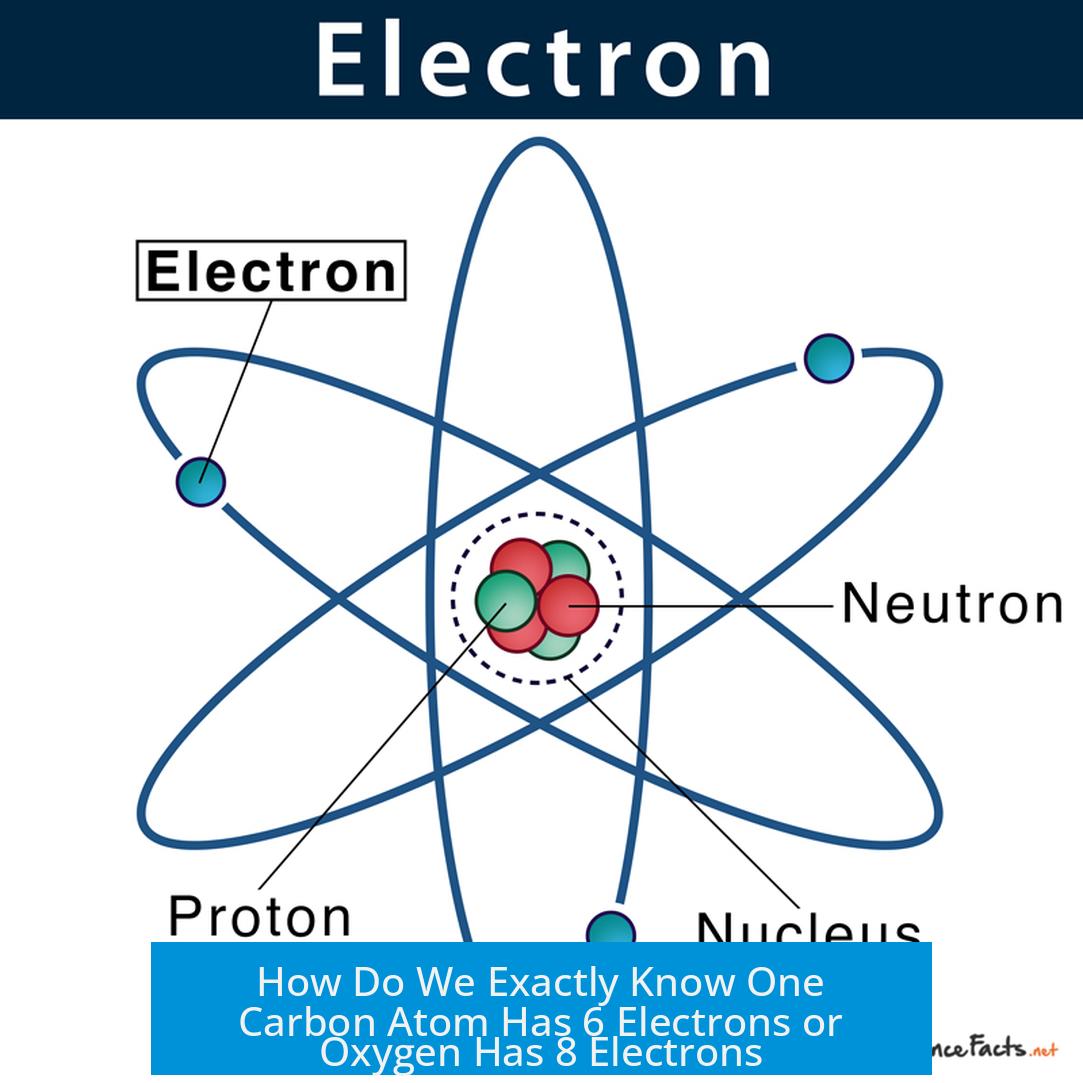 We know the exact number of electrons in atoms like carbon and oxygen primarily because the atomic number (Z)—the number of protons in the nucleus—is well defined and experimentally established. In neutral atoms, the number of electrons equals this atomic number. This insight comes from a combination of X-ray spectroscopy, periodic table organization, chemical measurements, and direct electron detection methods.
We know the exact number of electrons in atoms like carbon and oxygen primarily because the atomic number (Z)—the number of protons in the nucleus—is well defined and experimentally established. In neutral atoms, the number of electrons equals this atomic number. This insight comes from a combination of X-ray spectroscopy, periodic table organization, chemical measurements, and direct electron detection methods.
1. Atomic Number and Its Role in Defining Elements
The concept of atomic number (Z) represents the number of protons in an atomic nucleus. It essentially defines the identity of an element. For instance, carbon’s atomic number is 6, meaning it has 6 protons in its nucleus; oxygen’s atomic number is 8, signifying 8 protons.
This understanding arose from Henry Moseley’s work in the early 20th century. Moseley investigated the X-ray emission spectra of elements. He found a clear correlation: the frequency of emitted X-rays increased in a systematic way proportional to Z. This proved that Z is a physically meaningful count of protons, not just an arbitrary ordering number.
- Moseley’s experiment involved bombarding atoms with electrons to induce X-ray emissions.
- The X-ray energies correspond to electron transitions influenced by the nuclear charge.
- The direct link of X-ray energy to Z allowed precise identification of the proton count.
2. Neutral Atoms Have Equal Numbers of Protons and Electrons
Atoms of an element are electrically neutral when isolated or in elemental form. Since protons carry positive charge, this neutrality requires an equal number of negatively charged electrons to balance.
Therefore, carbon atoms, having 6 protons, must have 6 electrons to maintain neutrality. Similarly, oxygen atoms have 8 electrons to balance their 8 protons.
This rule is fundamental but has exceptions in ions, where atoms gain or lose electrons. For example, in sodium chloride (table salt), sodium atoms lose electrons to become positively charged ions, while chlorine gains electrons to form negative ions. However, in simple elemental form, neutrality holds.
3. Determining Atomic Number Using X-Ray Techniques and Periodic Table Position
Modern methods to determine atomic number include advanced X-ray spectroscopy and analysis of periodic table patterns. As Moseley showed, an element’s characteristic X-ray frequencies directly reflect Z. Scientists also correlate an element’s position in the periodic table, arranged by increasing Z, to confirm proton counts.
- Characteristic X-rays come from electron transitions between energy levels near the nucleus.
- The energy difference corresponds to Z-dependent nuclear attraction.
- Combined with periodic trends, this confirms each element’s atomic number precisely.
4. Atomic Weights and Chemical Evidence of Electron and Proton Counts
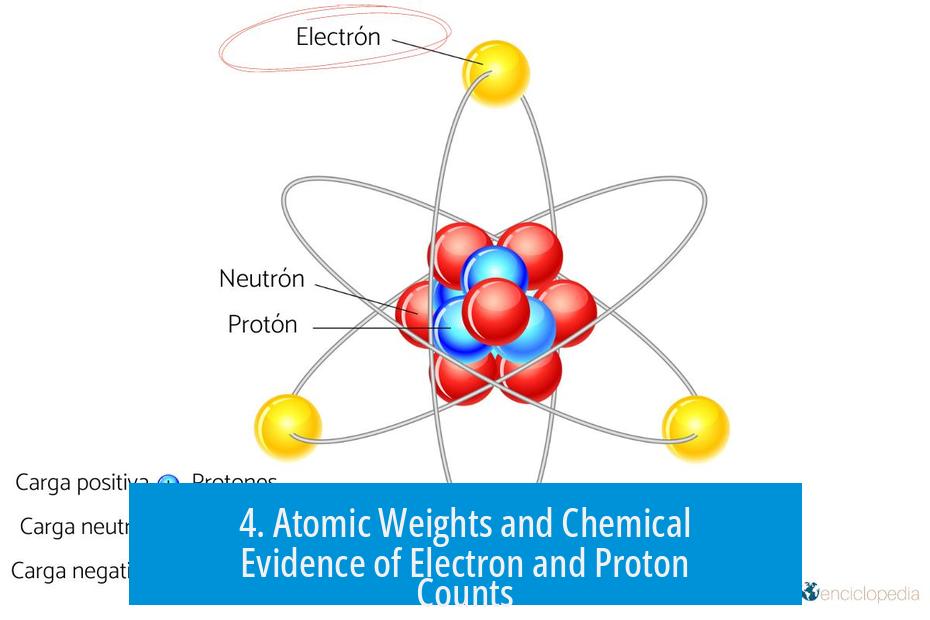
Atomic weights, established by centuries of chemical experimentation, support the atomic number concept. By carefully measuring reactant and product masses, scientists deduced the relative atomic masses of elements.
Hydrogen’s atomic weight serves as a baseline (approximately 1), and others scale accordingly—carbon around 12, oxygen near 16. These ratios align closely with the numbers of protons, reinforcing molecular composition theories.
| Element | Approximate Atomic Weight | Atomic Number (Z) |
|---|---|---|
| Hydrogen (H) | 1.008 | 1 |
| Carbon (C) | 12.01 | 6 |
| Oxygen (O) | 16.00 | 8 |
Although atomic weight includes neutrons as well, the proton count is closely linked to chemical properties, which depend on electrons.
5. Photoelectron Spectroscopy (PES) Confirms Electron Counts Experimentally
Photoelectron Spectroscopy (PES) is a direct experimental technique that probes the number and energy distribution of electrons in atoms.
PES utilizes high-energy X-rays to eject electrons from atoms. Each electron’s kinetic energy after ejection depends on the binding energy at its atomic orbital. Electrons closer to the nucleus are held more tightly and exit with less kinetic energy than outer electrons.
- By measuring kinetic energies of ejected electrons, scientists map electron energy levels.
- The number of peaks in the PES spectrum corresponds to the number of electrons in different orbitals.
- This method confirms that carbon has 6 electrons distributed in energy states, and oxygen has 8 electrons similarly organized.
PES data correlate with quantum mechanical predictions and reinforce the count of electrons matching the atomic number in neutral atoms.
6. Conceptual and Logical Foundations of Electron Number in Atoms
All experimental data converge on the premise that the atomic number equals the number of protons. Neutral atoms therefore have an equal number of electrons.
This relationship explains chemical behavior, bonding, and elemental properties. Electrons arrange in orbitals balancing positive charge, and unstable electron counts lead to chemical reactivity or ionic formation.
The atomic number thus serves as the fundamental atomic fingerprint, revealing the exact electron count in neutral atoms like carbon and oxygen.
Summary of How We Know the Electron Counts in Carbon and Oxygen
- Atomic number determined by Moseley’s work defines the number of protons in an element.
- Neutral atoms have equal numbers of electrons and protons.
- X-ray emission spectra provide precise atomic number measurements.
- Periodic table organization and atomic weights support electron-proton correspondence.
- Photoelectron Spectroscopy verifies electron numbers and energy distributions experimentally.
- Chemical neutrality and electron arrangement underpin elemental properties.
How Do We Exactly Know One Carbon Atom Has 6 Electrons or Oxygen Having 8 Electrons, for Example?
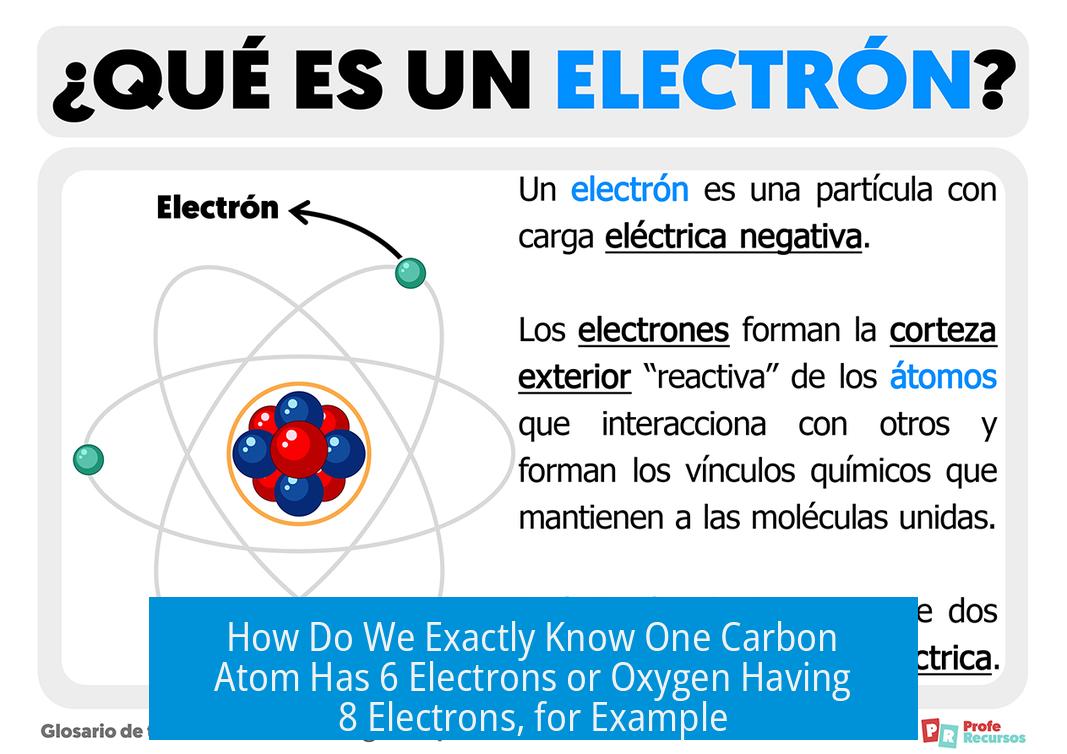
So, how do we *really* know that a carbon atom has exactly 6 electrons, or oxygen has 8?* You might think these are just numbers slapped onto the periodic table out of thin air. But science has done some serious detective work over the centuries to nail down these facts. Let’s dive into the fascinating story of how scientists concluded these electron counts—mixing historical breakthroughs, clever experiments, and clever logic.
Grab your lab coat—or just your curiosity—because we’ve got X-rays, atomic weights, and some mind-bending spectroscopy ahead!
The Atomic Number: Moseley’s Key Discovery
The journey starts with Henry Moseley, who, around 1913, cracked the code linking one invisible atomic property to something measurable. Instead of just guessing, Moseley measured the X-ray emission energies of different elements. He plotted these energies against an assigned number Z, known as the atomic number.
Moseley’s plot showed a tidy correlation: this “Z” wasn’t random. It represented the actual number of positive charges—protons—in the nucleus.
This discovery shook up chemistry. Suddenly, the atomic number was a real, countable thing. Elements were no longer just ordered by weight but by the count of protons. For carbon, that number is 6; oxygen has 8. And since the atomic number defines the element, it also sets the stage for the electron count.
Neutrality Means Electrons Match Protons
Now, you might be wondering: why do electrons have to be the same in number as protons? The answer lies in neutrality. Atoms in their elemental form don’t carry net charge—they are neutral.
Because oxygen has 8 protons (positive charges), elemental oxygen must have 8 electrons (negative charges) to balance out the electric charge.
So, it’s not just a random rule—it’s a necessary balance to be electrically neutral. Of course, atoms can lose or gain electrons becoming ions, but in typical elemental form, they are balanced like this.
Peeking Inside Atoms with X-rays and the Periodic Table
Knowing the atomic number isn’t guesswork; it’s backed up by X-ray techniques that confirm exactly how many protons are packed inside the nucleus.
Instruments measure the characteristic X-rays that atoms emit, and each element has a unique signature tied to its proton number.
The periodic table’s arrangement mirrors this, placing elements in order of increasing atomic number. This organization reveals the “secret code” to their electron counts.
The Weight of Evidence: Atomic Masses
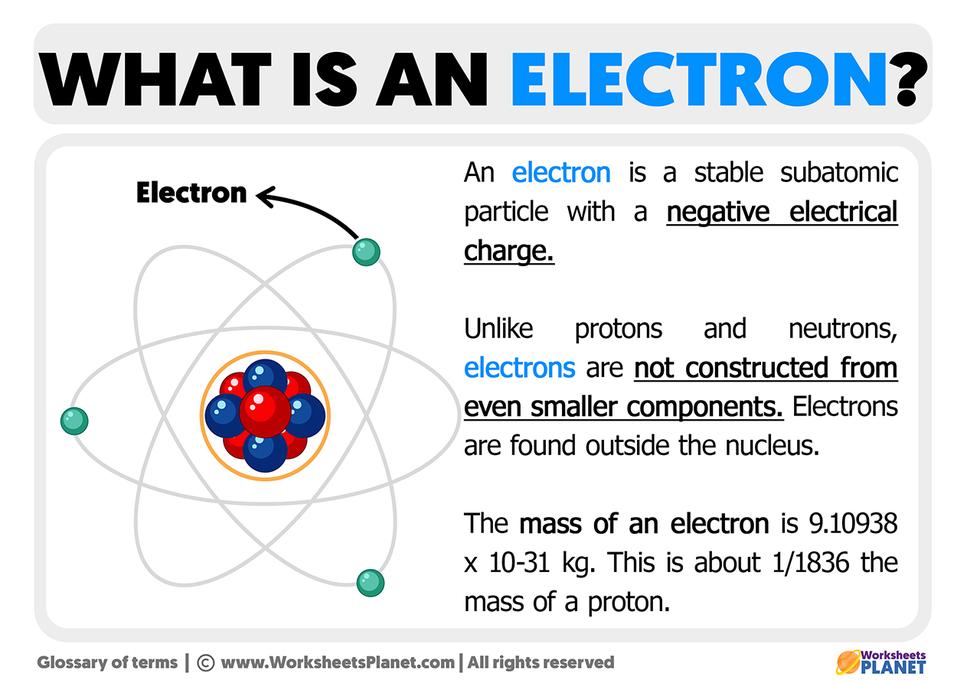
Chemists have been weighing stuff for centuries. That’s right, old-school balance scales played a vital role in confirming atomic theory.
By carefully measuring mass ratios of reactants and products in chemical reactions, scientists found consistent atomic weights that aligned with proton counts and indirectly with electron numbers.
For instance, hydrogen—the simplest atom—defines a baseline. Carbon weighs about 6 times as much as hydrogen; oxygen roughly 8 times. This proportionality backed up the idea of atomic numbers matching the fundamental particles inside atoms.
Though atomic weight isn’t exactly electron count, it’s a close cousin because electrons have negligible weight compared to protons. Still, these weight ratios provided an early foundation for precision in elemental identity.
Photoelectron Spectroscopy (PES): Catching Electrons in Action
Here comes the cool experimental evidence: Photoelectron Spectroscopy. This technique shoots X-rays at atoms to kick electrons right out of their cozy homes.
Electrons closer to the nucleus hold tighter and need more energy to leave. By measuring the speed and energies of ejected electrons, scientists map out how many electrons are at various energy levels.
PES creates a graphical “fingerprint” of electrons per atom. For carbon, you get a pattern confirming 6 electrons; for oxygen, 8 electrons. This direct window into the atomic world leaves little doubt about electron counts.
Putting It All Together: Logical, Experimental, and Historical Proof
So, how do we *exactly* know that a carbon atom has 6 electrons or oxygen 8? It’s a combination of:
- Moseley’s X-ray work: tying atomic number to protons.
- Neutrality of atoms: electrons balance out positive charges.
- Periodic table position: confirming atomic number order.
- Atomic weight experiments: chemical mass ratios aligning with theoretical counts.
- Photoelectron spectroscopy: visually confirming electron amounts at various energy levels.
Put simply: since the atomic number equals proton count, and neutral atoms must balance electrons with protons, carbon’s 6 protons mean 6 electrons, and oxygen’s 8 mean 8 electrons.
Why Does This Matter Above All?
If you ask yourself, “Why do we care?” here’s the kicker: electrons determine how atoms behave chemically. That count of electrons shapes the atom’s interactions, bonding, and the whole beautiful complexity of chemistry.
Stable chemical behavior hinges on electron arrangements—these depend on knowing precisely how many electrons orbit each atom’s nucleus.
Getting these numbers right is foundational for everything from understanding water molecules to designing new materials and medicines.
Final Thought: Science Isn’t Guesswork
It’s easy to glance at an atomic number and think, “Okay, number 6 means 6 electrons,” but science builds that certainty through layered experiments and logical deductions.
Every elemental bit of knowledge about atoms—from Moseley’s X-rays to the buzzing electrons seen in spectroscopy—works together like a well-oiled machine.
So next time you see that “6” for carbon or “8” for oxygen, remember it’s more than a number—it’s a proven fact standing on centuries of careful work.



Leave a Comment