How Does Orbital Hybridization Occur? And What Does 25% s Orbital Character Mean in sp3 Hybridization?

Orbital hybridization occurs when atomic orbitals on the same atom combine to form new hybrid orbitals. In the case of sp3 hybridization, one s orbital mixes with three p orbitals, creating four equivalent hybrid orbitals. Each sp3 orbital contains 25% s orbital character, indicating that one-quarter of its electron density arises from the s orbital and three-quarters from the p orbitals.
Understanding Orbital Hybridization
Hybridization as a Conceptual Model
Hybridization is a theoretical model chemists use to explain molecular bonding. It is not a physical process that can be observed directly. Instead, it helps rationalize why atoms form specific bond angles and shapes in molecules. For example, methane (CH4) has four identical bonds arranged tetrahedrally. Pure atomic orbitals alone cannot explain this geometry.
The model mixes atomic orbitals mathematically, allowing predictions of bonding and molecular shapes. It maintains the octet rule and provides insight into bonding without contradicting quantum mechanics.
Why Hybridization Is Needed

Carbon’s valence shell has one 2s orbital and three 2p orbitals oriented perpendicularly. If orbitals did not hybridize, bonds would form at angles of 90°, reflecting the p orbital directions. However, methane’s bonds are approximately 109.5°, suggesting orbitals adjust to new shapes. Hybridization describes this adjustment.
The Mechanism of sp3 Hybridization
Atomic Orbitals Involved
- Carbon’s valence orbitals: 1 × 2s orbital, 3 × 2p orbitals (px, py, pz)
- Total orbitals mixed: one s and three p orbitals
These four atomic orbitals combine to produce four equivalent sp3 hybrid orbitals. Each has the same energy and shape, which is distinct from the original s and p orbitals.
Formation of Four Equivalent Orbitals
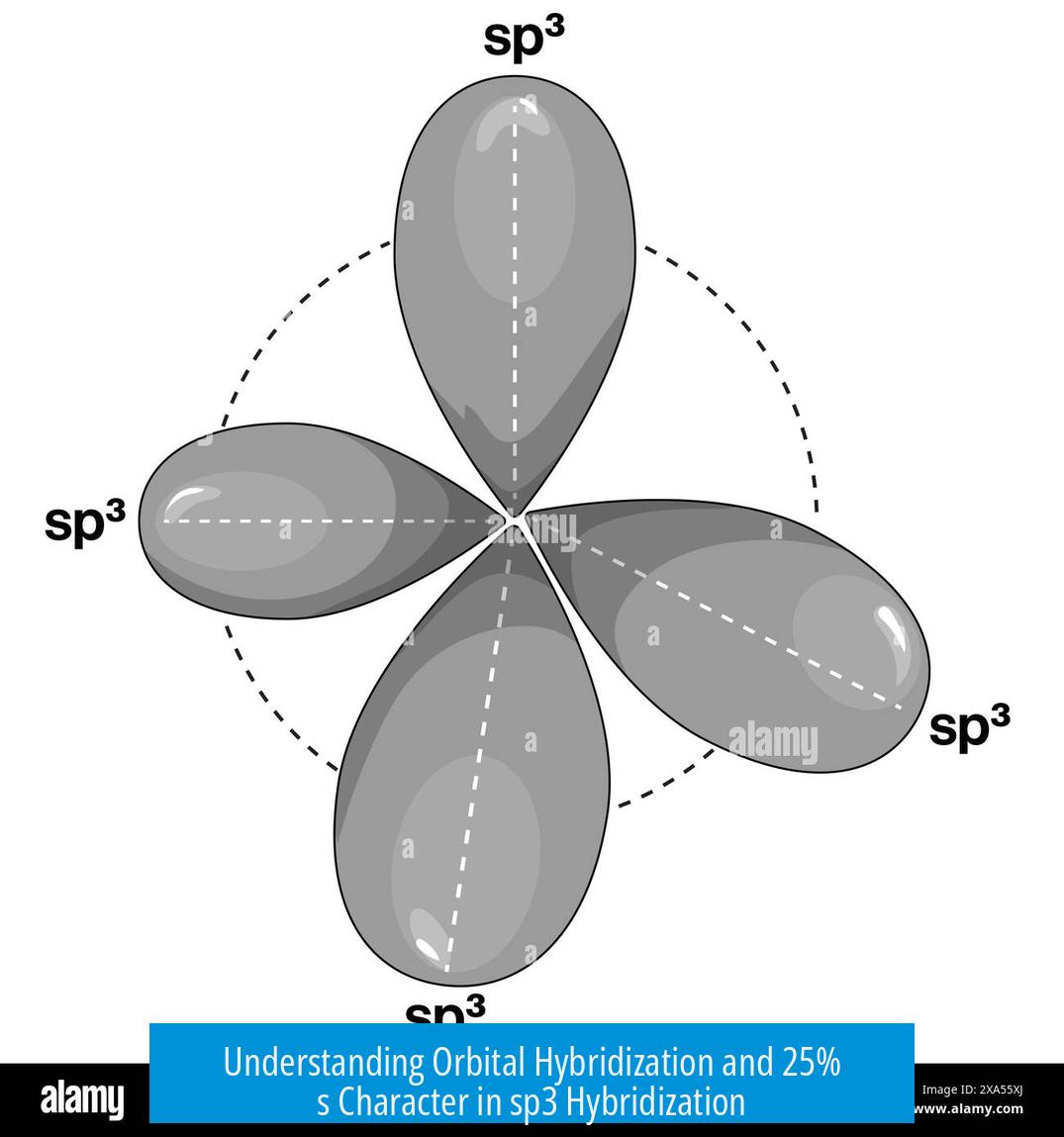
Hybridization blends the s and p orbitals as a linear combination. The name “sp3” signifies one s orbital plus three p orbitals joined. This process creates four lobed orbitals directed toward the corners of a tetrahedron.
Each sp3 hybrid orbital points in a specific direction, facilitating the formation of four identical bonds with bond angles near 109.5°. The combination lowers orbital energy and increases overlap with bonded atoms.
Meaning of 25% s Orbital Character in sp3 Hybridization
Quantitative Composition of sp3 Orbitals
| Orbital Type | Number of Orbitals Combined | Total Percentage Contribution |
|---|---|---|
| s orbital | 1 | 25% |
| p orbitals | 3 | 75% |
Each sp3 orbital contains exactly 25% s orbital character. This means a quarter of the orbital’s wave function derives from the s orbital’s spherical distribution. The remaining 75% comes from the directional p orbitals.
Mathematical Representation: Linear Combination
Hybrid orbitals are best described as weighted sums of atomic orbitals. This mathematical approach uses coefficients indicating each atomic orbital’s contribution. For sp3 orbitals, the coefficient for the s orbital corresponds to 25%, and for p orbitals, combined coefficients total 75%.
“The molecular orbital that has that tetrahedral sp3 shape is a sum of 25% s orbital plus the character from the three p orbitals depending on orientation.”
Effect on Orbital Shape and Bonding
- Higher s character makes orbitals more spherical and lower in energy.
- Higher p character increases directional properties of orbitals, influencing bond angles and reactivity.
- In sp3, the balance creates orbitals with tetrahedral geometry suitable for bonding four atoms.
This specific composition gives carbon-based molecules such as methane their stable, well-defined structures.
Additional Aspects of Hybridization
Hybridization Model Limitations
Hybridization is a simplification. Real molecules exhibit electron distributions more complex than hybrid models suggest. Electronic interactions and quantum mechanics provide more precise descriptions.
The model remains widely used due to its predictive power about molecular geometry and bonding behavior.
Energetic and Structural Implications
- Hybrid orbitals lower overall orbital energy compared to pure atomic orbitals.
- Increased orbital overlap enhances covalent bond strength.
- Bond angles predicted by hybridization closely match experimental values.
- Small deviations occur in real molecules, but sp3 remains an effective approximation.
Key Takeaways
- Hybridization blends atomic orbitals on one atom to form hybrid orbitals with new shapes and energies.
- In sp3 hybridization, one s and three p orbitals produce four equivalent orbitals with tetrahedral geometry.
- Each sp3 orbital consists of 25% s character and 75% p character, quantifying orbital composition.
- The model explains molecular geometry and bonding patterns better than using isolated atomic orbitals.
- Hybridization lowers orbital energies and maximizes overlap, stabilizing molecular bonds.
- It is a conceptual tool, not a directly observable physical phenomenon.
What triggers orbital hybridization in atoms like carbon?
Hybridization occurs when atomic orbitals mix to form new hybrid orbitals. Carbon blends one 2s and three 2p orbitals to create four equivalent sp³ hybrids.
How do sp³ hybrid orbitals differ from pure s or p orbitals?
Sp³ orbitals combine one s and three p orbitals. Each hybrid orbital has 25% s character, giving it partial s orbital shape and energy but with directional features from p orbitals.
What does having 25% s orbital character in sp³ mean chemically?
It means each sp³ hybrid orbital contains one quarter s orbital and three quarters p orbitals. This ratio affects molecular shape and bond angles, producing tetrahedral geometry near 109.5°.
Why can’t hybridization be directly observed?
Hybridization is a theoretical model. We use it to explain molecular geometries and bonding patterns, although actual electron distributions are more complex and not directly seen.
How does hybridization help predict molecular shapes?
By mixing s and p orbitals, hybridization creates orbitals with geometries that match observed bond angles. For example, sp³ orbitals explain methane’s tetrahedral bonds instead of simple 90° p orbital angles.




Leave a Comment