How Does Salt Neutralize Acid?
Salt does not neutralize acid directly. In typical situations, common salt (sodium chloride) does not chemically react with acids to neutralize them. Instead, salt affects the perception of acidity in foods by altering taste sensations without changing the acid’s chemical nature.
Salt Does Not Chemically Neutralize Acid
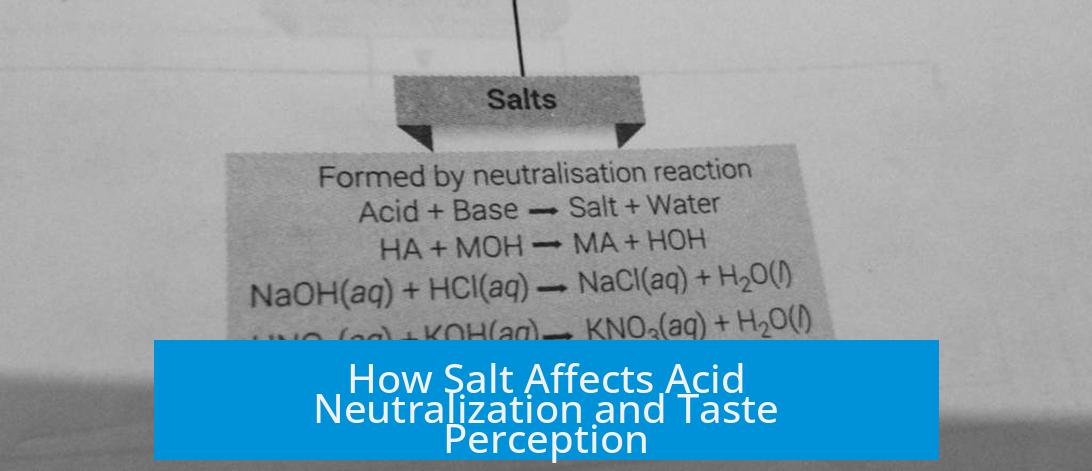
Common table salt, or sodium chloride, lacks the chemical properties necessary to neutralize acids. Neutralization usually involves a reaction between an acid and a base that produces water and a salt. Since salt is neither a base nor reacts with acid in this way, it does not neutralize acid in food or other contexts. For instance, salt sprinkled on apples does not reduce their acidity chemically.
Only Some Salts Can Neutralize Acids
Certain salts, especially those derived from weak acids and bases, can neutralize acids. For example, baking soda (sodium bicarbonate) neutralizes vinegar (acetic acid) by reacting to produce carbon dioxide, water, and sodium acetate. However, table salt does not have this capability.
How Salt Affects Taste Perception
Salt acts as a flavor enhancer. It reduces the perception of sourness and acidity by masking acidic tastes. When salt is added to apples, the fruit may taste sweeter or less tangy. This effect arises from how salt influences taste buds, rather than any acid neutralization. Thus, the acid remains chemically intact while its intensity feels diminished.
No Chemical Reaction Between Salt and Acid in This Context
The interaction between salt and acid on foods like fruit does not involve chemical changes but sensory perception. Any salty taste on fruit is due to salt’s presence on the surface, not a neutralization process. Sometimes, washing fruit with water removes salt, and the apparent reduction in acidity is due to dilution or rinsing, not a chemical neutralization by salt.
| Substance | Neutralizes Acid? | Mechanism |
|---|---|---|
| Sodium Chloride (Table Salt) | No | Alters taste perception only |
| Sodium Bicarbonate (Baking Soda) | Yes | Reacts with acid to form salt and water |
Other Considerations
- A very salty taste in fruit might suggest contamination or other factors, not neutralization.
- If fruit tastes unusually salty or spicy, consider allergy or sensory irregularity.
- Water used to rinse items can reduce acidity by dilution, not salt chemically neutralizing acid.
Key Takeaways
- Table salt does not neutralize acid through chemical reaction.
- Salt influences acidity perception by affecting taste buds.
- Only certain salts like baking soda physically neutralize acids.
- Rinsing with water can reduce acidity via dilution, not via salt.
- Unusual salty taste in fruit may indicate other issues, including allergies.
Does salt chemically neutralize acids?
No, table salt (sodium chloride) does not chemically neutralize acids. It simply does not react with acidic substances to reduce their acidity.
Can any salts neutralize acids?
Some salts, like baking soda (sodium bicarbonate), can neutralize acids through a chemical reaction. However, regular kitchen salt does not have this effect.
Why does salt seem to reduce the sourness of acid in food?
Salt affects taste perception by enhancing flavors. It can make acidic food taste less sour by increasing sweetness and masking acidity, but it doesn’t change the acid level.
Is the neutralization effect due to salt or water in food preparation?
The neutralization is often due to water washing away acid, not because of salt. Water can dilute acids and reduce their impact on taste.
Could a salty taste in fruit indicate a problem?
If a fruit tastes unusually salty, it might not be related to salt addition but could be a sign of an allergy or other issue. Fruits typically do not have a salty flavor naturally.



Leave a Comment