How to Understand Character Tables and Irreducible Representations

Understanding character tables and irreducible representations (irreps) requires a solid grasp of group theory and its mathematical foundations. These tools are central to inorganic and theoretical chemistry for analyzing molecular symmetry, although modern computational methods often handle much of the work. A focused approach rooted in mathematical principles and practical examples aids comprehension and application.
Foundations in Group Theory
Character tables arise from group theory, where a group is a set of symmetry operations that combine according to specific rules. Every molecular point group corresponds to such a group. For instance, if you know a few symmetry operations, the group rules allow you to generate all remaining operations systematically. This structure gives character tables their power and consistency.
Group theory introduces key concepts relevant to representations:
- Representations describe how symmetry operations transform molecular orbitals or vibrational modes.
- Irreducible representations (irreps) are the smallest symmetry components, indivisible under the group operations.
- Reduction breaks a given representation into a sum of irreps.
- The Great Orthogonality Theorem ensures orthogonality among irreps, providing mathematical rigor.
Mathematical textbooks focusing on group and representation theory, rather than simplified chemistry treatments, better clarify these principles. Concepts such as the Schmidt operator help derive projectors onto irreps, deepening understanding.
Role of Character Tables in Chemistry
Character tables summarize symmetry properties of molecular point groups. They list symmetry elements, classes of symmetry operations, and characters (traces) of irreps with respect to these operations. In practical chemistry, these tables serve to:
- Determine which molecular orbitals or vibrations correspond to specific symmetry types.
- Predict selection rules for spectroscopic transitions.
- Guide construction of molecular orbitals consistent with symmetry.
- Classify molecular vibrations in vibrational spectroscopy.
Despite their usefulness, most modern chemistry leverages computational software to apply character tables implicitly, reducing the need for manual use. However, learning how to read and use these tables remains vital for a deep grasp of molecular symmetry.
Learning Through Examples and Patterns
Irreps are often denoted by symbols such as A, B, E, and T. Examples clarify their meaning:
- A irreps represent symmetric behavior under the principal rotation axis.
- E and T irreps represent doubly and triply degenerate symmetry types, respectively, often seen in molecules with higher symmetry.
- Labels like g (gerade) and u (ungerade) indicate inversion symmetry (even or odd).
Consider common molecules:
| Molecule | Point Group | Irreps present |
|---|---|---|
| Methane | Td | E, T |
| Cobalt hexaquo | Oh | E, T, g, u |
Working through concrete examples helps internalize the meaning and usage of different irreps. Practice enables recognition of patterns across groups such as C3h vs. C3v and D3h vs. D3v, facilitating memorization and intuition.
Using Mathematical Formulas
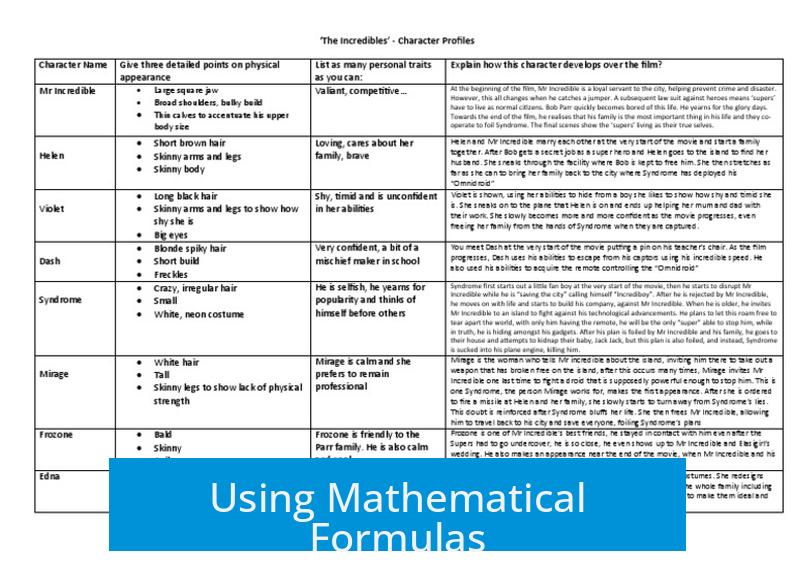
There is a formulaic approach to generating irreducible representations and reducing any representation into irreps. This involves:
- Calculating characters for each symmetry operation by tracing matrix representations.
- Applying orthogonality relations derived from the Great Orthogonality Theorem.
- Utilizing projection operators to isolate parts of a representation corresponding to irreps.
This systematic approach demystifies irreps and clarifies why reducing representations is necessary.
Common Challenges
Even advanced students find certain areas difficult:
- Normal mode analysis requires assigning vibrational motions to irreps, which can be complex.
- Chirality and its impact on symmetry complicates character table interpretations in polyatomic molecules.
- Visualizing degenerate irreps (E and T) can be challenging without molecular examples.
Continuous practice and revisiting foundational group theory help overcome these obstacles.
Recommended Resources and Support
For those wishing to deepen their understanding, the early chapters of Cotton’s Chemical Applications of Group Theory provide valuable step-by-step examples.
Additionally, online communities such as r/chemhelp offer assistance with targeted questions.
Key Takeaways
- Character tables are rooted in group theory and symmetry operations form groups with defined rules.
- Irreducible representations break down complicated symmetry behaviors into fundamental units.
- Proper mathematical treatment, including group and representation theory, clarifies these concepts.
- Practice with specific molecules and point groups improves understanding.
- Computational tools often perform character table applications today, but learning manual methods is essential.
- Reliable textbooks and community resources support ongoing learning efforts.
What is the best way to start understanding character tables?
Begin by studying group and representation theory. A good math textbook clarifies what representations are, why they reduce, and important theorems. This solid foundation helps grasp character tables more naturally.
How do character tables connect to group theory?
Character tables represent groups and obey group rules. If you identify some symmetry operations, you can generate the others. Recognizing these patterns simplifies understanding the whole table.
How do irreducible representations (irreps) relate to molecular symmetry?
Irreps correspond to symmetries like rotation or reflection. For example, ‘A’ irreps relate to symmetry along the main axis. Molecules like methane (Td) and cobalt hexaquo (Oh) show common irreps such as E and T.
Is there a way to derive irreducible representations if I don’t memorize them?
Yes, a formula exists to generate irreps from group data. This lets you find them mathematically without memorization, though practice helps improve speed and intuition.
Why is it still hard to work with character tables and irreps after learning?
Concepts like normal modes and chirality remain challenging. Real-world examples and continual practice with problems improve understanding but some topics are naturally tough.
Where can I get help with specific character table questions?
Online communities like r/chemhelp offer targeted answers for tricky problems. Asking detailed questions there can clarify specific doubts when self-study hits a wall.



Leave a Comment