Understanding Tungsten and Its Density

I got 1Kg of Tungsten today! Tungsten is notable for its density of 19.25 g/cm3. This value is much higher than that of lead, which has a density near 11.34 g/cm3, and is close to gold at 19.32 g/cm3. This makes tungsten one of the densest metals commonly available, giving it special physical properties and applications.
Name and Origin
The name “tungsten” comes from the Danish words “tung” meaning heavy and “sten” meaning stone. This reflects its characteristic high density and heavy nature.
Physical Characteristics
- Density: 19.25 g/cm3
- Melting Point: 3422°C, one of the highest among metals
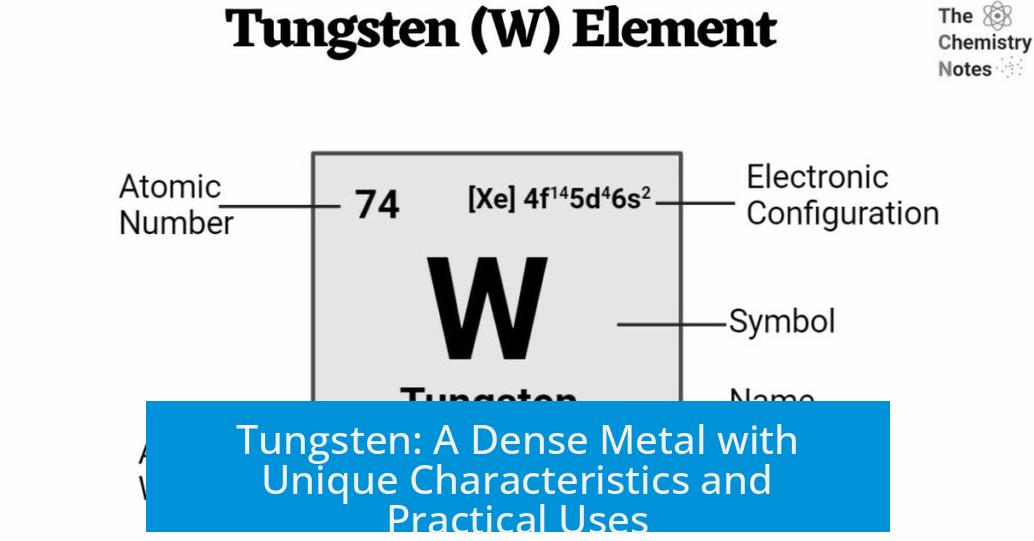
- Atomic Number: 74
These properties closely match those of wolfram, another name for tungsten. Sometimes confusion arises because “wolfram” is used interchangeably with tungsten, sharing nearly identical physical characteristics.
Practical Uses of Tungsten
Tungsten’s high density and melting point make it suitable for various applications:
- Military: Used as micro-shrapnel in Dense Inert Metal Explosive (DIME) munitions.
- Industrial: Utilized in cutting tools and heavy machinery due to its hardness.
- Lifestyle: Used to make whiskey stones, offering a dense material that chills drinks without dilution.
Its density also allows tungsten to serve as a material substitute in fake gold bars. Counterfeiters use tungsten because it closely mimics gold’s weight and feel.
Handling and Limitations
Tungsten is difficult to work with. It cannot be easily cut or cast using conventional methods. This limits its use in custom shapes or hobbyist projects but reinforces its strength and durability.
Health and Safety Considerations
While tungsten itself is relatively non-toxic, concerns exist about long-term exposure and kidney health if ingested. However, using tungsten for whiskey stones poses minimal risk as the metal is inert and does not dissolve in typical conditions.
Summary
- Tungsten is extremely dense, nearly as dense as gold, and much denser than lead.
- Its Danish name means “heavy stone,” reflecting its physical nature.
- Tungsten and wolfram are the same element with matching properties.
- Common uses include military ordinance, industrial tools, and chilled drink stones.
- It is hard to machine or cast, limiting ease of handling.
- Health risk from casual use is minimal, but ingestion concerns warrant caution.
What does the name “Tungsten” mean?
Tungsten comes from Danish words: “tung” meaning heavy and “sten” meaning rock.
Is Tungsten the same as Wolfram?
Yes, Tungsten and Wolfram are the same element. They share the same density, melting point, vapor pressure, and atomic number.
Why is Tungsten often mistaken for gold in fake bars?
Because Tungsten’s density (19.25 g/cm³) is nearly the same as gold’s, making it ideal to mimic gold bars.
Can Tungsten be easily cut or cast into shapes?
No, Tungsten is hard to cut or cast due to its physical properties. It requires special tools or methods for shaping.
Are there any health risks from handling Tungsten objects, like whiskey stones?
Tungsten is generally safe to handle, but ingesting particles or prolonged exposure might harm kidneys. Using it as whiskey stones poses minimal risk if clean and intact.




Leave a Comment