Why Do Non-Polar Compounds Dissolve in Non-Polar Solvents?
Non-polar compounds dissolve in non-polar solvents mainly because of the entropy of mixing and similar London dispersion forces (LDF) acting between molecules. These factors favor a random, uniform distribution of molecules rather than phase separation, driving solubility despite the weak intermolecular forces involved.
Understanding the Driving Forces Behind Non-Polar Solubility
Unlike polar compounds, where dipole-dipole interactions and hydrogen bonding explain solubility, non-polar compounds lack strong electrostatic attractions. Instead, their dissolution depends on subtle but significant forces combined with thermodynamic principles.
- Entropy of Mixing: The increase in disorder when molecules mix drives solubility.
- London Dispersion Forces (LDF): Weak, induced dipole interactions present in all molecules facilitate mutual attraction between non-polar species.
- Size and Molar Volume: Molecular dimensions influence how well compounds pack and interact.
Entropy of Mixing as the Key Factor
Entropy measures the degree of randomness in a system. When a non-polar solute spreads evenly in a non-polar solvent, the overall disorder increases. This change favors a mixed state over phase separation.
For example, nitrogen and oxygen gases mix spontaneously in air despite weak interactions. Similarly, non-polar molecules like carbon tetrachloride (CCl4) dissolve in hexane because the system gains disorder. The entropy gain offsets the relatively small energy changes involved.
The entropy effect becomes clearer when imagining a flask with solute and solvent molecules. If all experience similar weak London dispersion forces, no strong preference exists to cluster separately. Random distribution yields higher entropy, promoting mixing.
London Dispersion Forces Facilitate Like-Like Molecular Interaction
Non-polar molecules interact primarily via London dispersion forces. These forces arise from temporary fluctuating dipoles in electron clouds, causing weak attraction between molecules.
While individually weak, LDFs are universal and cumulative. Non-polar solute and solvent molecules experience similar magnitudes of dispersion forces. This similarity creates a “like dissolves like” scenario that favors mixing.
- LDF strength depends on polarizability and molecular surface area.
- Non-polar solvents and solutes avoid penalizing energy penalties upon mixing.
- The even distribution of weak attractions supports solubility.
Though not as strong or directional as dipole interactions or hydrogen bonds, dispersion forces suffice to hold non-polar molecules together in solution when entropy also favors spreading.
Molecular Size and Molar Volume Affect Solubility
Size influences how molecules fit together. Smaller non-polar molecules often mix better in non-polar solvents due to easier packing and greater entropy changes upon mixing.
The Flory-Huggins interaction parameter (χ) is a model that quantifies compatibility. It combines effects of molecular interactions and molar volumes to predict solubility behavior.
| Factor | Effect on Non-Polar Solubility |
|---|---|
| Molecular Size | Smaller molecules mix more freely; larger molecules may face steric hindrance. |
| Molar Volume | Similar molar volumes improve packing and reduce unfavorable voids. |
| Interaction Similarity | Like-like London dispersion strengths improve miscibility. |
For example, chloroform and acetone, which have moderate polarity and small size, can dissolve in non-polar solvents due to these combined factors.
Why Solvation Energy Alone Does Not Explain Non-Polar Solubility
Heat of solvation quantifies the energy change when molecules interact with solvent. In ionic or polar compounds, this strongly affects solubility. However, it often fails to predict non-polar solubility.
Take calcium carbonate (CaCO3)—despite favorable solvation heat in water, it remains poorly soluble because ionic bonds are stronger in the solid lattice than interactions with water molecules.
In contrast, carbon tetrachloride dissolves in hexane not because of high solvation energy, but because both molecules experience similar dispersion forces and entropy gain dominates.
Comparison to Oil-Water Immiscibility
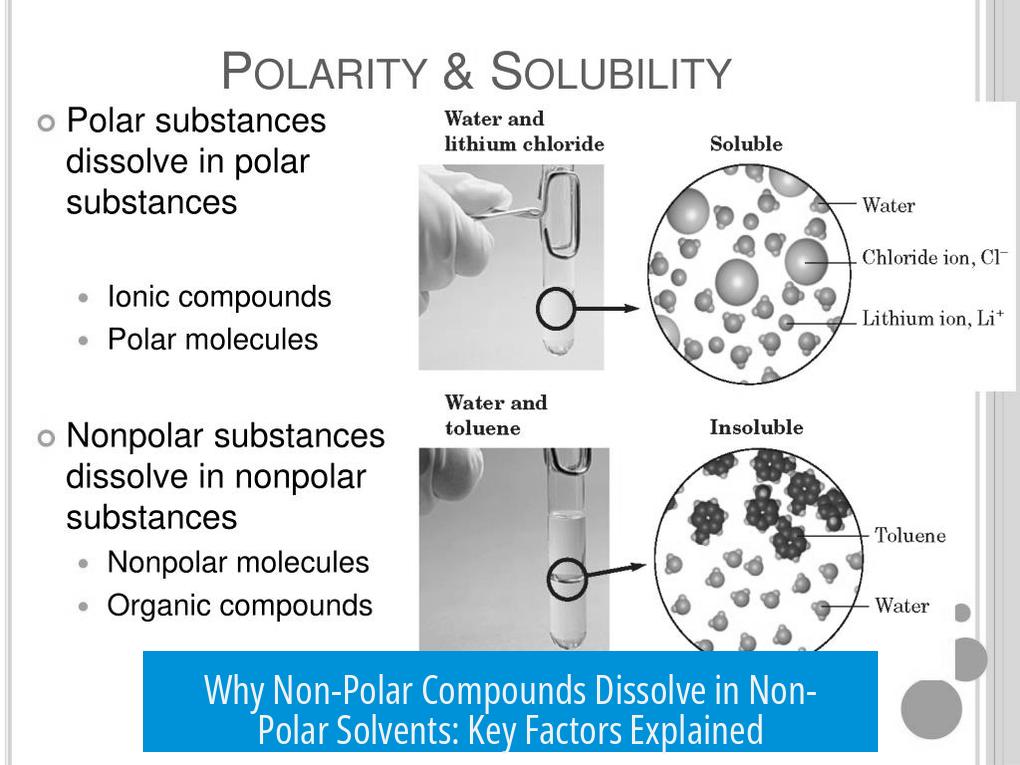
Oil and water phase separate due to drastic differences in polarity and intermolecular forces. Water strongly hydrogen bonds with itself and excludes non-polar molecules to minimize disruption.
This is an entropy-driven effect. Water molecules gain freedom when oil molecules cluster, decreasing unfavorable interactions.
In non-polar solvent systems, no such mismatch exists. Instead, similar dispersive forces and increased entropy encourage mixing, unlike oil-water immiscibility.
Core Reasons for Non-Polar Solubility Explained
- Non-polar solute and solvent molecules share weak but compatible London dispersion forces.
- Entropy of mixing favors molecular randomness, offsetting weak interaction energies that might resist dissolution.
- Molecular size and molar volume affect packing efficiency and solution stability.
- Heat of solvation has limited influence; similar intermolecular force profiles guide miscibility.
- Non-polar systems contrast with polar-ionic ones, where strong electrostatic or hydrogen-bond interactions dominate.
Summary of Key Takeaways
- Entropy drives non-polar compounds to dissolve in non-polar solvents by increasing disorder in the mixture.
- London dispersion forces enable compatible, weak attractions between solute and solvent molecules.
- Molecular size and molar volume influence packing and interaction uniformity.
- Heat of solvation plays a minor role in non-polar solubility compared to polar or ionic cases.
- Non-polar solubility exemplifies “like dissolves like” through similar intermolecular forces and thermodynamics.




Leave a Comment