Understanding the Appearance of Iron(II) Sulfate Heptahydrate
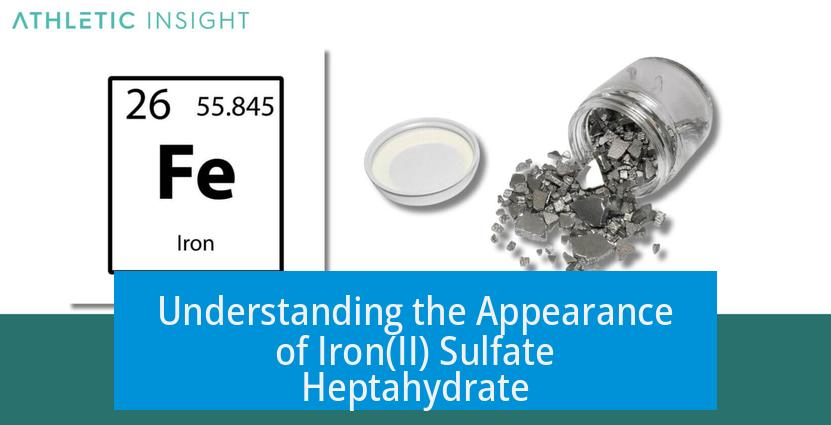
Iron(II) sulfate heptahydrate often appears discolored because it oxidizes easily, changing from its original pale green or blue-green color to brown. This color change occurs due to the oxidation of Iron(II) (Fe2+) ions into Iron(III) (Fe3+) ions upon exposure to air. The oxidation reaction is a common and spontaneous process influenced by environmental oxygen.
Oxidation of Iron(II) Sulfate
Iron(II) ions in ferrous sulfate readily oxidize to Iron(III) ions when exposed to air. This transformation results in a visible color shift:
- Initially, Iron(II) sulfate heptahydrate displays a pale green or blue-green shade.
- Upon oxidation, the compound develops a brownish hue linked to Iron(III) formation.
- Ferrous salts tend to oxidize slowly but become noticeably brown when oxidation advances.
Such oxidation explains why an opened or improperly stored jar of Iron(II) sulfate may appear brown instead of its typical blue-green color.
Stability Conditions for Iron(II) Sulfate
Iron(II) sulfate is more stable in certain environments. Acidic conditions reduce oxidation rates, maintaining Fe2+ ions longer.
For applications requiring stable Iron(II) ions, Mohr’s salt ((NH4)2Fe(SO4)2) offers enhanced stability. Mohr’s salt combines iron(II) sulfate with ammonium sulfate, resulting in a more oxidation-resistant compound, suitable for precise chemical work.
| Compound | Color | Stability | Oxidation Tendency |
|---|---|---|---|
| Iron(II) sulfate heptahydrate | Blue-green (fresh), Brown (oxidized) | Less stable when exposed to air | Oxidizes easily to Iron(III) |
| Mohr’s salt | Blue-green | More stable (acidic environment) | Less prone to oxidation |
Summary of Key Points
- Iron(II) sulfate heptahydrate oxidizes in air, turning brown due to Fe2+ to Fe3+ conversion.
- Proper storage and acidic conditions help maintain its original blue-green color.
- Mohr’s salt provides a more stable form of Iron(II) sulfate for laboratory use.
Why does Iron(II) sulfate heptahydrate sometimes appear brown instead of its usual color?
Iron(II) sulfate heptahydrate can oxidize to Iron(III) when exposed to air. The brown color indicates this oxidation has occurred. Normally, it should be blue or green.
What causes Iron(II) sulfate heptahydrate to oxidize quickly?
Exposure to air accelerates the oxidation of Iron(II) to Iron(III). Ferrous salts easily oxidize, especially if stored in large, open containers.
How can I keep Iron(II) sulfate stable and prevent oxidation?
It remains more stable in acidic conditions. Alternatively, use Mohr’s salt ((NH4)2Fe(SO4)2), which is more resistant to oxidation under normal storage.
Is the color change reversible if Iron(II) sulfate heptahydrate starts to oxidize?
No, once it oxidizes to Iron(III), the change is permanent. The brown coloration means the compound no longer contains Iron(II) in the same form.
Are there visual signs that indicate Iron(II) sulfate heptahydrate has oxidized?
Yes, the compound turns brown instead of its usual blue-green color. The presence of air in storage containers often leads to this visible change.




Leave a Comment