Is -1 pH a Thing?
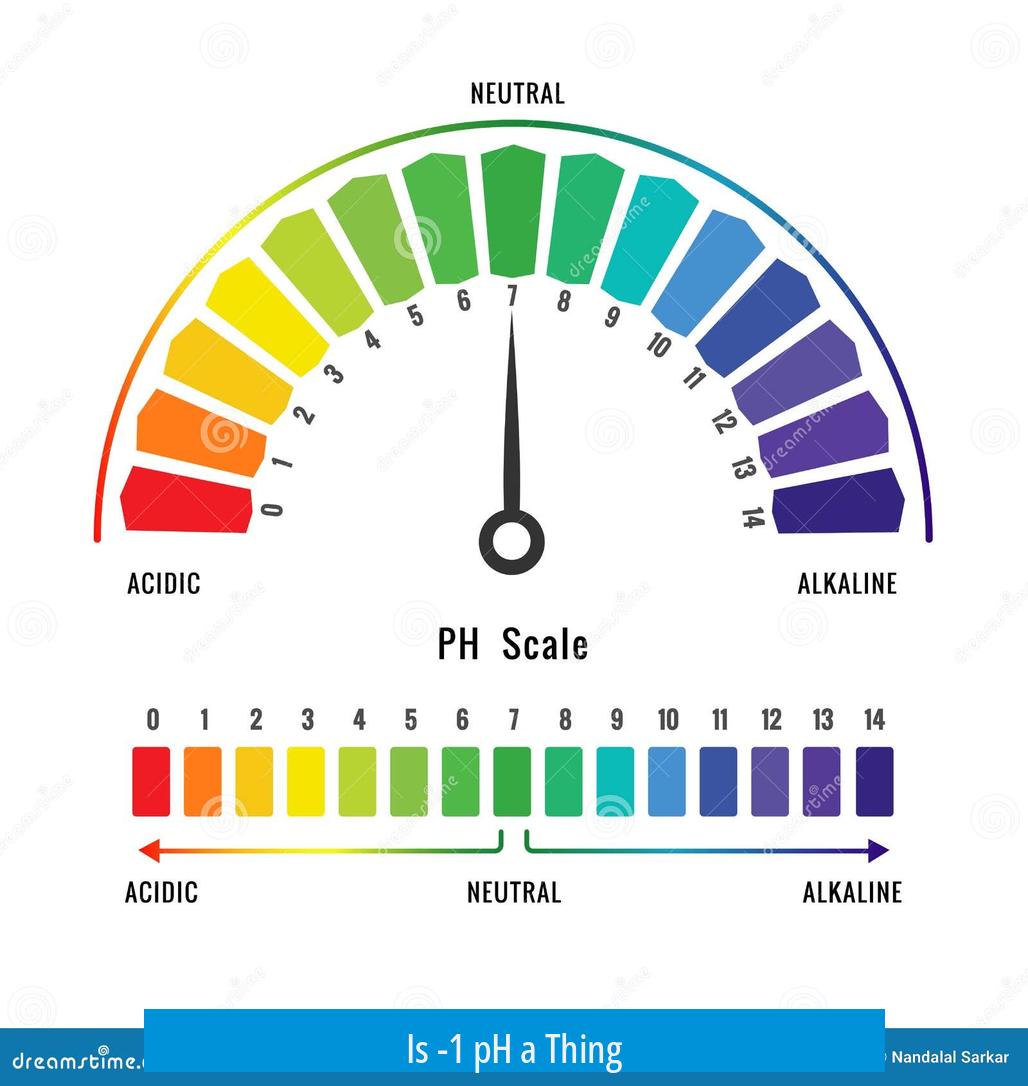
The pH scale can indeed have values below zero, including -1 pH, as it is based on the negative logarithm of hydrogen ion activity. While most practical scenarios show pH values between 0 and 14, highly concentrated acidic solutions can have pH values less than zero, including -1 and even lower.
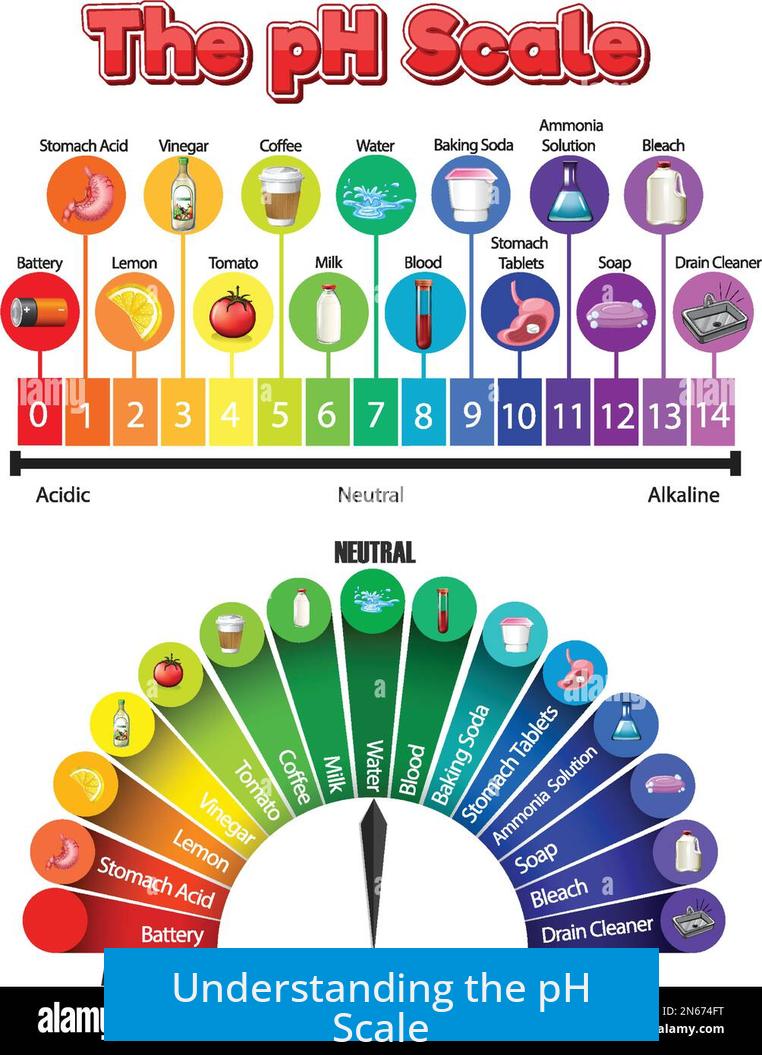
Understanding the pH Scale
The pH scale measures acidity or alkalinity by the formula:
pH = -log [H+]
This means pH is the negative logarithm of the hydrogen ion concentration, or more precisely, the hydrogen ion activity in water. Because the log function extends indefinitely in both directions, pH does not have a fixed lower or upper limit.
The usual range of 0 to 14 arises from typical aqueous solutions at 25°C. It corresponds roughly to hydrogen ion concentrations from 1 molar (pH 0) to 10-14 molar (pH 14). However, this is a practical range rather than a strict boundary.
When Can pH Be Negative?
If the hydrogen ion concentration in a solution exceeds 1 mole per liter, the pH will be negative. For example, a 10 M solution of HCl corresponds to:
- [H+] = 10 M
- pH = -log(10) = -1
Therefore, a pH of -1 is theoretically valid.
In reality, strong acids like concentrated hydrochloric acid can reach and even surpass this point. For instance, a 11.9 M HCl solution has a measured pH around -2.8, considering that hydrogen ion activity is higher than the concentration due to ion interactions.
Limitations of pH Measurement and Activity
Measured values deviate because pH is based on hydrogen ion activity rather than concentration. Activity factors account for the actual effective concentration considering interactions within highly concentrated solutions.
As acids become very concentrated, the solution behaves less like a simple aqueous medium. This affects water’s ability to dissociate and the accuracy of glass electrode pH meters, which become less reliable below about pH 0.5. Despite this, reproducible measurements down to around -4 have been achieved using specialized techniques.
Superacids and Extreme Acidity
There exist superacids with pKa values far below zero. For example:
- Benzene sulfonic acid has a pKa near -7.
- Fluoroantimonic acid (HSbF6) has an effective pH estimated as low as -28 to -30.
These acids demonstrate that pH values less than -1 are chemically possible, though not commonly encountered in everyday labs. They often exist in non-aqueous or highly specialized conditions.
Real-World Examples of Negative pH
Extremely acidic environments such as mine waters have recorded pH values less than zero. The Iron Mountain mine in California has solutions with reported pH as low as -3.6, along with extremely high metal ion and sulfate concentrations.
Such measurements are rare and typically require careful calibration. Nonetheless, they confirm that negative pH values are not just theoretical but can occur naturally.
Practical and Conceptual Considerations
- Most biochemical and environmental processes operate within the pH range 0–14.
- Negative pH values correspond to conditions where water no longer behaves as an ideal solvent.
- Measuring very low pH values requires specialized electrodes and careful calibration.
- The classical pH scale is convenient but has limitations for extremely concentrated or non-aqueous systems.
- For highly acidic solutions, alternative acidity functions such as the Hammett acidity function (H0) provide more accurate characterization.
Common Misconceptions About Negative pH
- A common myth holds that pH must lie strictly between 0 and 14; this is false.
- Negative pH values do not imply instability or danger beyond normal concentrated acid hazards.
- The logarithmic nature of pH means extreme values are mathematically possible but not often encountered.
Summary of Key Points
- The pH scale is a negative logarithmic scale and can have values below zero, including -1.
- A solution with hydrogen ion concentration over 1 M will have negative pH.
- Concentrated acids like 10 M HCl can have a pH near -1; 11.9 M HCl can reach approximately -2.8.
- Negative pH is measurable but requires specialized methods, especially below pH 0.5.
- Superacids and some natural acidic waters exhibit strongly negative pH values.
- The typical 0 to 14 range is practical, not absolute.
- Alternative acidity scales are preferred for highly concentrated or non-aqueous systems.
Can pH values be negative, like -1 pH?
Yes. pH is a negative logarithm of hydronium ion concentration. If [H⁺] = 10 M, the pH equals -1. Highly concentrated acids can have pH values below zero.
How is a pH below zero possible chemically?
It happens because the pH formula is -log of H⁺ activity. Extremely high proton concentrations, above 1 M, yield negative pH. For example, 11.9 M HCl has a pH around -2.8 due to high proton activity.
Are there real examples of negative pH in nature or industry?
Yes. Some mine waters show pH as low as -3.6. Superacids can have pH values near -30. Measurement becomes tricky below pH 0.5, but it is reproducible down to about -4.
Does a negative pH mean water is no longer behaving normally?
Often, yes. At very low pH, the solution may stop acting like typical water. Water activity drops and dissociation is incomplete, making the pH scale less reliable in these conditions.
Are pH values above 14 or below 0 common in everyday chemistry?
No. Most pH measurements range from 0 to 14. Values outside this range occur in extreme or highly concentrated solutions. For normal purposes, the scale between 0 and 14 suffices.




Leave a Comment