Is Ba(OH)2 Partially or Completely Soluble in Water?
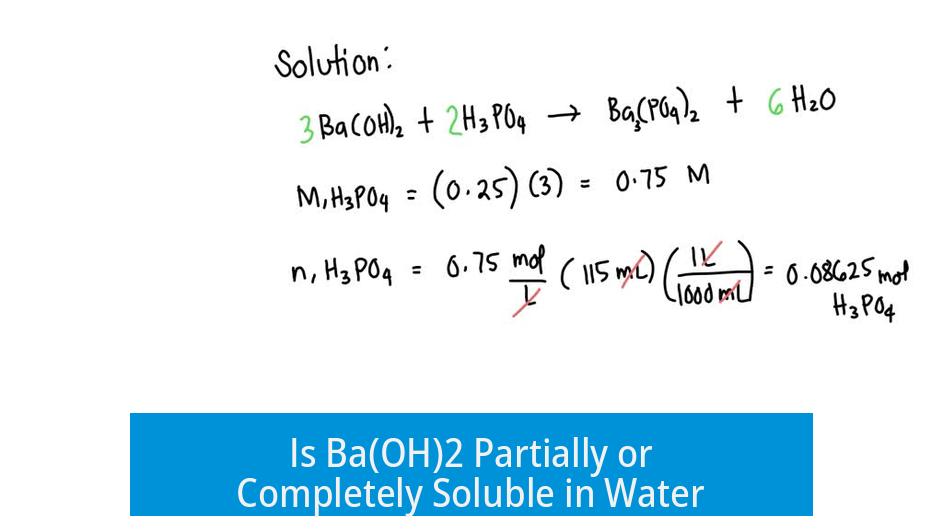
Barium hydroxide (Ba(OH)2) is considered soluble in water and almost completely ionizes, but describing it as either “partially” or “completely” soluble can be misleading.
Clarifying Solubility Terms
The usual categories “partially soluble” or “completely soluble” do not fully capture the solubility behavior of Ba(OH)2. Instead, it is better to state that Ba(OH)2 is soluble and dissociates extensively into ions in water.
Ionization and Dissociation in Water
Barium hydroxide is a strong Arrhenius base. Upon dissolving, it largely dissociates into Ba2+ and OH- ions. This near-complete ionization means it produces a high concentration of hydroxide ions, lending it strong basic properties.
Practical Solubility Features
- Ba(OH)2 dissolves readily in water at room temperature with a notable solubility of about 3.89 g/100 mL (20°C).
- Its high ionic dissociation differentiates it from less soluble barium compounds, such as barium carbonate (BaCO3), which does not dissolve appreciably.
- Old or exposed Ba(OH)2 samples may contain barium carbonate from reaction with atmospheric CO2. This insoluble carbonate can be removed by filtration when preparing a solution.
Applications Highlighting Solubility
Barium hydroxide solution serves as a traditional method for measuring carbon dioxide content in gases. When CO2 bubbles through the Ba(OH)2 solution, it forms insoluble barium carbonate. This precipitate is filtered and weighed, benefiting from barium’s large atomic weight to accurately quantify CO2.
Summary
- Ba(OH)2 is soluble and almost completely ionizes in water.
- Terms “partially” or “completely” soluble are not precise for Ba(OH)2.
- Old samples may contain insoluble barium carbonate, removable by filtration.
- The strong solubility and ionization make Ba(OH)2 useful in analytical applications, especially CO2 detection.



Leave a Comment