Is Glycine Molecule a Polar or Nonpolar Molecule?
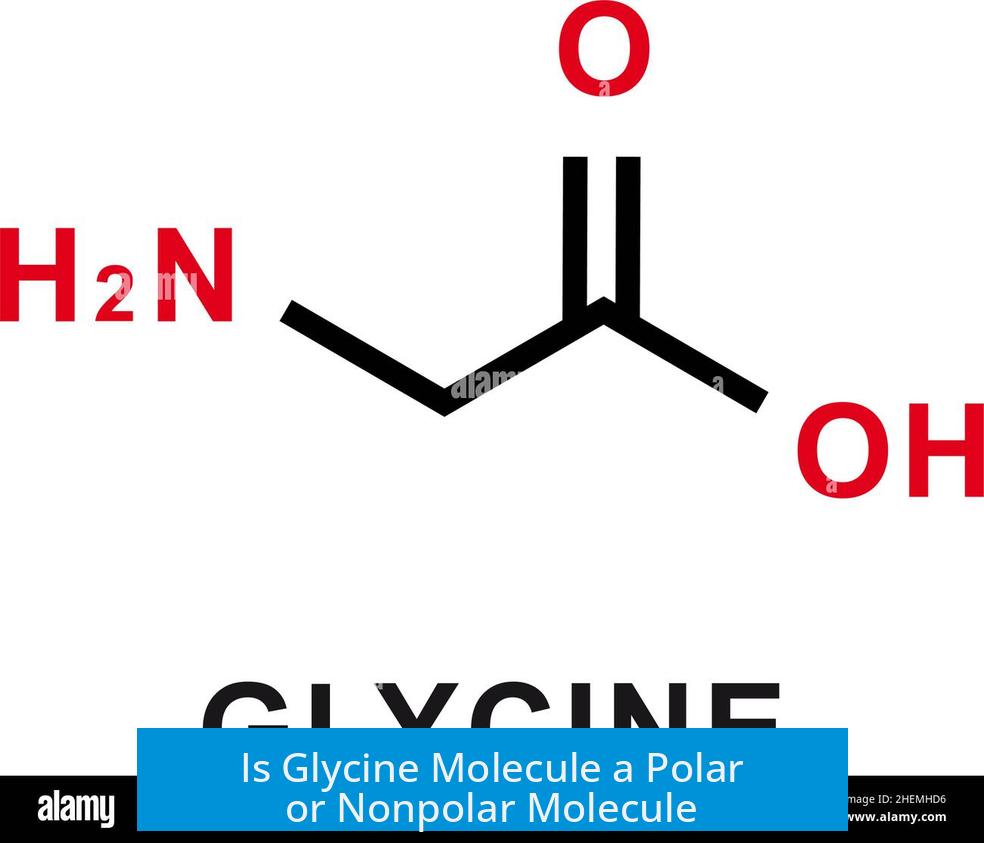
The glycine molecule is polar overall, despite having a nonpolar side chain. This is because the molecule contains polar functional groups contributing significant polarity, outweighing the nonpolar characteristic of its side chain.
Understanding Molecular Polarity
Polarity in molecules exists on a spectrum. It depends on the presence and arrangement of polar bonds and the molecule’s geometry. Polar molecules have uneven distribution of electron density, resulting in partial positive and negative charges.
Polarity in Amino Acids
Amino acids generally tend to be polar molecules. This polarity arises mainly from their amine (-NH2) and carboxylate (-COOH) groups, which contain electronegative atoms and can ionize. These functional groups create dipoles and enable interactions like hydrogen bonding.
When discussing amino acid polarity, often the focus is on the side chain (R-group). Side chain polarity varies widely among different amino acids, affecting how proteins fold and interact.
The Polarity of Glycine
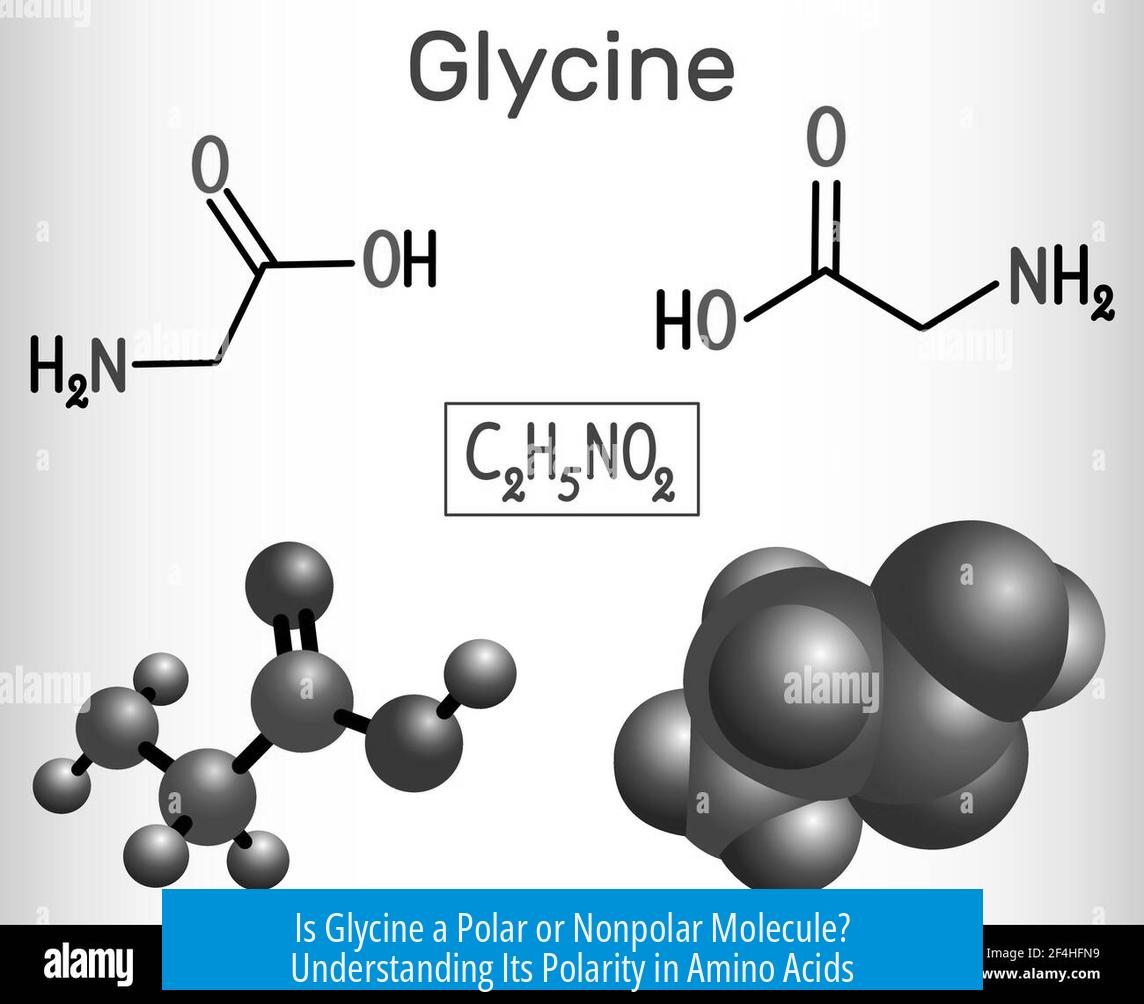
- Functional Groups: Glycine has an amine group and a carboxyl group, both polar and ionizable.
- Side Chain: The side chain of glycine is a single hydrogen atom, which is nonpolar.
Despite its nonpolar side chain, the dominant polar groups make glycine an overall polar molecule. The molecule’s ionic nature, especially the charged amine and carboxylate groups at physiological pH, increases its polarity significantly.
Assessing Polarity Through Functional Groups
To determine glycine’s polarity, one examines its functional groups rather than side chain alone. The amine group (–NH2) and carboxyl group (–COOH) introduce regions of charge and polarity. Glycine’s shape and charge distribution cause it to interact well with polar solvents like water.
| Component | Polarity |
|---|---|
| Amine group (–NH2) | Polar |
| Carboxyl group (–COOH) | Polar |
| Side chain (Hydrogen) | Nonpolar |
Key Takeaways
- Glycine is a polar molecule overall due to its polar functional groups.
- The nonpolar side chain of glycine is a single hydrogen atom.
- Polarity depends more on functional groups than on the side chain.
- Amino acids typically have polar properties because of amine and carboxyl groups.
Is glycine a polar molecule overall?
Yes, glycine is polar. The presence of both an amine group and a carboxylate group creates polarity in the molecule.
Does glycine have a nonpolar part?
Yes, glycine’s side chain is nonpolar. However, this does not make the whole molecule nonpolar.
How does glycine’s polarity compare to other amino acids?
Like most amino acids, glycine is generally polar due to its functional groups. Its polarity mainly arises from the ionic nature of these groups.
What determines the polarity of glycine?
Look at glycine’s functional groups—the amine and carboxylate. These ionic parts give it polar properties despite its nonpolar side chain.
Is polarity in molecules always yes or no?
Polarity exists on a spectrum. Molecules can have areas that are polar and others that are nonpolar, like glycine with its mix of functional groups.



Leave a Comment