Is NH3+ Acidic or Basic?

NH3+ as such is not a commonly recognized species in chemistry; it most likely refers to the ammonium ion, NH4+, which is weakly acidic. Ammonia (NH3) itself is a base, not an acid.
Clarifying NH3+ Identity
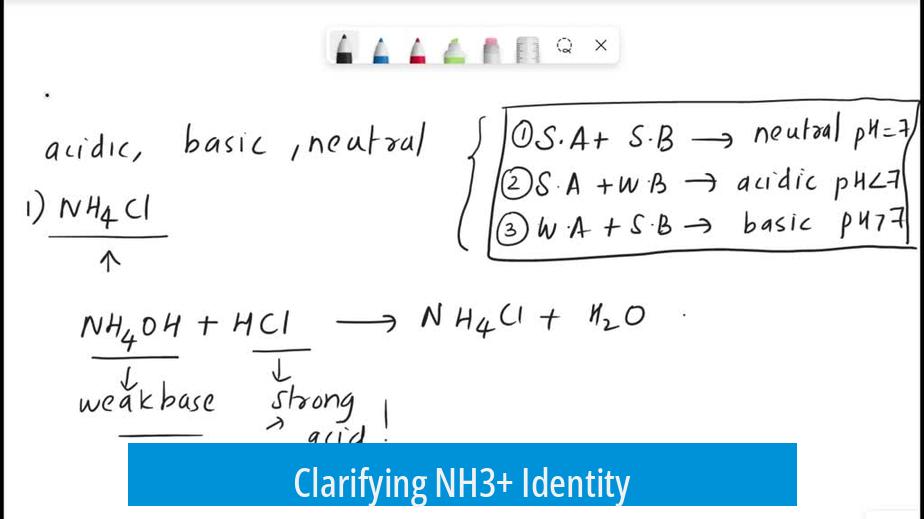
The ion denoted as NH3+ suggests removing one electron from ammonia (NH3), creating a positively charged radical cation. This species is highly unstable and uncommon in typical chemistry contexts. Most likely, the intended species is the ammonium ion, NH4+.
Ammonium ion forms when ammonia accepts a proton (H+):

NH3 + H+ → NH4+
NH3+ radical ions react quickly, often capturing radicals or electrons to form NH4+.
Acidity and Basicity Comparison
| Species | Nature | Character |
|---|---|---|
| NH3 (Ammonia) | Molecule | Basic, accepts protons |
| NH4+ (Ammonium Ion) | Ion | Weakly acidic, can donate protons |
| NH3+ (Radical Cation) | Unstable Ion | Not typically encountered, reacts rapidly |
Ammonia (NH3) acts as a base because it can donate a lone pair to accept a proton.
Ammonium ion (NH4+) is the conjugate acid of ammonia. It shows slight acidity by donating a proton under certain conditions. Its acidity is weak compared to strong acids but enough to influence equilibrium in aqueous solutions.

Contextual Nature of Acid-Base Behavior
Acidic or basic properties depend on the comparative chemical environment. For example, NH4+ is acidic when compared with NH3 but may be neutral compared to stronger acids.
In aqueous solution, ammonia accepts a proton to form NH4+, which can then release a proton back to water, displaying weak acidity for NH4+ and weak basicity for NH3.
Key Takeaways
- NH3+ radical cation is not a stable or common species.
- NH4+ (ammonium ion) is weakly acidic.
- NH3 (ammonia) is a basic molecule.
- Acid-base nature depends on the chemical environment and relative comparison.
- Ammonium’s acidity is weak, reflecting its role as the conjugate acid of ammonia.
Is NH3+ Acidic or Basic? Unraveling the Mystery of This Curious Ion
Have you ever stumbled upon the notation NH3+ and scratched your head wondering, “Is this thing acidic or basic?” Welcome to the quirky world of nitrogen chemistry! Spoiler alert: NH3+ as a standalone ion isn’t your typical chemical party guest—it’s rare, fleeting, and reactive. But by diving deeper, you’ll see why its behavior revolves around a more familiar cousin: the ammonium ion, NH4+.
So, let’s decode this puzzle, clear up misconceptions, and understand the acid-base personality of NH3+, NH4+, and ammonia (NH3) itself. Ready? Let’s bust some chemistry myths!
First Things First: What Exactly Is NH3+?
Here’s where things get tricky. When you see NH3+, fingers start pointing… Is it a typo? A radical cation? Or did someone mean NH4+, the well-known ammonium ion?
Well, NH3+ isn’t a stable character in everyday chemistry scenes. This ion represents an ammonia molecule that lost an electron, making it a radical cation. Radical cations are rarely isolated on their own because they react eagerly with nearly any nearby species, especially radicals.
Imagine NH3+ crashing into a hydrogen radical (H•). What’s the product? The famous ammonium ion (NH4+). This reaction transforms that elusive NH3+ into something everyone recognizes and understands well—NH4+.
“NH3+ + H• → NH4+”
The takeaway? When analyzing acidity or basicity, chemists tend to focus on NH4+, not NH3+ radical cations, because NH3+ simply doesn’t hang around long enough to matter.
Understanding Acidity and Basicity of NH3, NH3+, and NH4+
So, what about the acid-base personalities of these species?
- NH3 (Ammonia) – A classic base. It has a lone pair of electrons on nitrogen ready to grab a proton. Ammonia typically acts as a weak base in water and many other solvents.
- NH4+ (Ammonium ion) – The protonated form of ammonia. Now, this guy is an acid, but only weakly acidic. In water, it can donate a proton back to ammonia or water due to its positive charge and polar N–H bonds.
- NH3+ (Radical cation) – Not truly acidic or basic in the conventional sense, as it’s highly reactive and fleeting. It quickly converts to NH4+ in the presence of typical radicals.
So, in plain English: ammonia is a base, ammonium is a weak acid, and NH3+ is mostly a chemical ghost.
Can Something Be Both Acidic and Basic? Context Matters!
Here’s a nugget you won’t hear often: whether a molecule is acidic or basic depends on the company it keeps. In chemistry, “acidic” or “basic” isn’t carved in stone but measured against other substances.
E.g., water shows up as both an acid and a base in different reactions.
You might ask: how does this concept apply to our nitrogen-containing molecules?
Ammonium ion (NH4+) is a weak acid compared to its base ammonia (NH3). However, if you compare ammonium with a stronger acid like hydrochloric acid, ammonium acts as a base.
So if context changes, the roles can flip! This perspective helps avoid rigid labels and deepens your chemical intuition.
Why Does This Matter? Real-World Implications
Understanding the acidic or basic nature of NH4+ and NH3 is crucial in many fields:
- Environmental Chemistry: Ammonia and ammonium ions influence soil pH and nutrient availability.
- Biochemistry: Nitrogen species like ammonia and ammonium play roles in metabolic pathways and nitrogen balance.
- Industrial Applications: Synthesis of fertilizers and cleaning agents relies on the correct handling of acidic/basic forms of nitrogen compounds.
Knowing that NH3+ is a fleeting radical and not a stable acid or base helps avoid confusion when you see it in advanced research or radical chemistry contexts.
Breaking It Down: Summary Table
| Species | Type | Acid/Base Character | Notes |
|---|---|---|---|
| NH3 | Molecule | Weak base | Has lone pair; accepts protons. |
| NH4+ | Ion (ammonium) | Weak acid | Protonated form of NH3; donates protons. |
| NH3+ | Radical cation (rare) | Reacts instantly (not acid/base) | Quickly forms NH4+ with radicals. |
Final Thoughts: What Would You Say About NH3+?
So, next time you bump into NH3+ in a chemical formula or a quiz, you can confidently say: “This isn’t a stable acid or base on its own; it’s a fleeting, reactive species that quickly turns into ammonium, which is weakly acidic.”
And if someone tries to challenge you with this question, you’ll already be one step ahead!
Don’t chemicals love to keep us on our toes?
Curious about other radical ions or want to dive deeper into nitrogen chemistry? Stay tuned, keep questioning, and remember—every molecule has a story, even if it’s as short-lived as NH3+!
Is NH3+ a stable ion in chemistry?
NH3+ is not commonly found as a stable ion. It often reacts quickly with radicals to form NH4+. It is generally considered an intermediate, not a stable species.
Is the NH3+ ion acidic or basic?
NH3+ does not have clear acidic or basic properties by itself because it reacts rapidly. However, the related ammonium ion (NH4+) is weakly acidic, while ammonia (NH3) is basic.
How does NH4+ differ from NH3 in terms of acidity?
NH4+ is weakly acidic because it can donate a proton. In contrast, NH3 acts as a base since it can accept protons.
Can NH3+ act as an acid or base depending on the context?
Acidity or basicity depends on the species it interacts with. NH3+ quickly forms NH4+, so its acidic or basic nature is not well defined alone.



Leave a Comment