Is Solubility a Chemical or Physical Property?
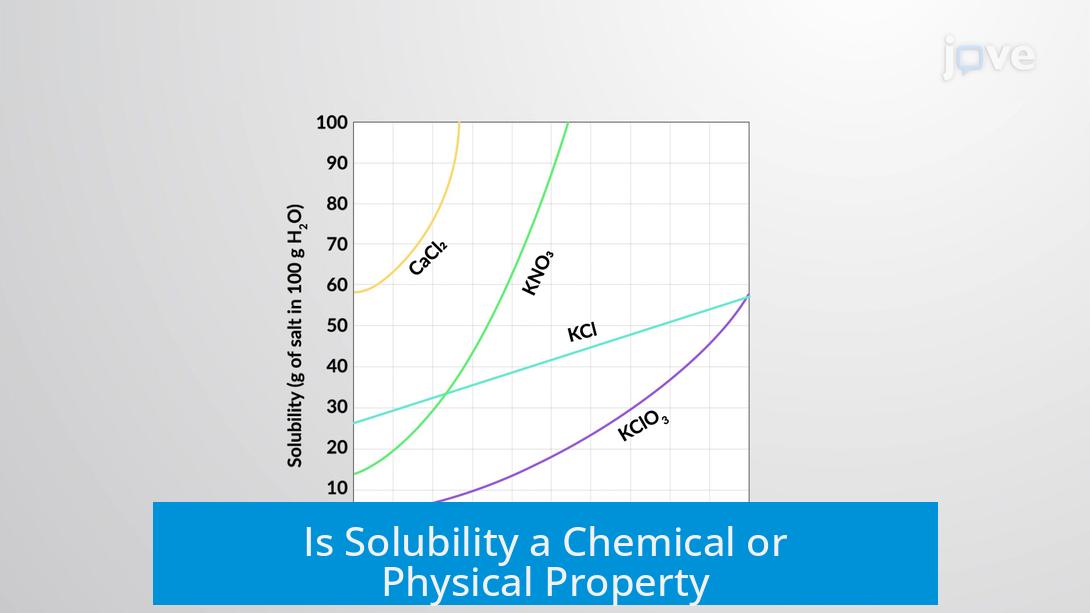
Solubility is primarily considered a physical property because it describes how a substance dissolves in a solvent without changing its chemical identity. It involves the interaction between solute and solvent molecules, such as salt dissolving in water. Despite complex interactions like forming metal-aquo complexes, the solute usually remains chemically unchanged and can be recovered.
Understanding Solubility
- Solubility is the ability of a substance (solute) to dissolve in another (solvent).
- No new substances form in the process; solute molecules or ions disperse evenly within the solvent.
- Because the original substance can be retrieved without chemical alteration, solubility ranks as a physical property.
- Exceptions exist like metal ions that form coordination complexes with water molecules.
Is Dissolving Salt in Water a Physical or Chemical Change?

Dissolving salt (sodium chloride) in water involves a chemical process known as ionic dissociation. The ionic lattice of the salt breaks apart into sodium (Na+) and chloride (Cl−) ions, which disperse throughout the water.
Details of the Dissolving Process
- The ionic bonds between Na+ and Cl− ions cleave when salt dissolves.
- Water molecules surround the free ions, stabilizing them through ion-dipole interactions.
- This separation and stabilization of ions is a chemical change because bonds are broken.
- Despite this, no new substances are formed; the ions remain sodium and chloride in solution.
Reconciling Physical and Chemical Aspects of Dissolution
The distinction lies in how chemical changes are defined. Bond cleavage counts as a chemical transformation, as seen in salt dissociation. But since the ions retain their identity and can recombine, the overall result behaves like a physical change in many contexts.
| Aspect | Solubility | Dissolving Salt in Water |
|---|---|---|
| Type of Property/Change | Physical property (generally) | Chemical change (ionic dissociation) |
| Molecular Interaction | Physical mixing with solvent | Breaking ionic bonds to form ions |
| Recoverability | Solute remains unchanged and can be recovered | Ions can recombine upon removing solvent |
Key Points
- Solubility is mainly a physical property since it does not alter the chemical nature of the solute.
- Dissolving salt in water is a chemical change due to ionic bond breaking and ion formation.
- The process involves both physical mixing and chemical bond changes.
- The distinction depends on the level of chemical interaction considered.
Is Solubility a Chemical or Physical Property? And Is Dissolving Salt in Water a Physical or Chemical Change?

We often toss the terms “chemical” and “physical” around when talking about properties and changes in matter. But where exactly does solubility fit? And when salt dissolves in water, are we witnessing a physical or chemical change?
In short: solubility is generally considered a physical property, yet dissolving salt in water involves a chemical change. Let’s unpack this fascinating nuance.
Imagine dropping table salt (sodium chloride) into a glass of water. The salt seems to disappear. Magic? Nope, chemistry.
Understanding the Basics: Chemical vs. Physical Properties
Chemical properties describe how a substance changes its identity—its atoms rearranged, new bonds formed, old bonds broken. Physical properties, by contrast, deal with characteristics that don’t alter the substance’s fundamental identity: color, melting point, density, or yes—solubility.
Now, solubility sounds straightforward. If a substance dissolves in a solvent, it shows solubility. But is solubility a chemical or physical property? To answer this, we need to get a bit deeper.
Solubility: The Devil’s in the Details
Typically, solubility is treated as a physical property. Why? Because, in most cases, the dissolved substance remains chemically unchanged, just “spread out” among the solvent molecules. Drop salt in water, stir, and the salt seemingly vanishes. However, if you evaporate the water, the same salt crystals reappear, unchanged. No new substance formed, no atoms rearranged. Physical change, right?
Not so fast.
There’s more complexity lurking behind the scenes. Salt dissolving involves ionic dissociation. Sodium chloride’s tightly bound crystal lattice breaks apart into sodium ions (Na+) and chloride ions (Cl−). This separation is more than a simple physical mixing. The salt’s ionic bonds break, and ions become solvated—or surrounded—by water molecules.
Some metallic ions don’t just float freely; they form specific entities called metal-aquo complexes. In these, water molecules coordinate chemically with metal ions, forming new coordination bonds—a chemical interaction.
So solubility sits on a spectrum: it’s mostly physical, with a sprinkling of chemical flavor.
What About Dissolving Salt in Water? Physical or Chemical Change?
Here is where things get clearer—but also more intriguing.
Dissolving salt in water is a chemical change. That’s because the salt’s ionic lattice breaks apart into separate sodium and chloride ions. Remember, a chemical change involves breaking bonds and creating new interactions. The ionic bonds holding Na+ and Cl− together in solid form break. Those ions become independent, surrounded by water molecules.
This is a crucial point: while the chemical composition of the sodium and chloride ions remains the same, the structure and bonding state changes. That qualifies as a chemical change, even if the individual substances do not convert into entirely new molecules.
In plain words, the salt molecules don’t just mix physically—they separate chemically into their parts.
A Practical Perspective: Why Does This Distinction Matter?

You might wonder why distinguishing solubility as a chemical or physical property is important. Here are some reasons:
- Recoverability: If a salt dissolves physically, you can recover it unchanged. This matters in desalination and water treatment industries.
- Predicting behavior: Knowing whether dissolution involves ionic dissociation helps chemists predict conductivity, reactivity, and interactions in solutions.
- Chemical processes: Industries leveraging metal-aquo complexes (like catalysis, pharmaceuticals) depend on understanding these subtle chemical interactions.
So the next time you add salt to water, consider this: You’re not just mixing; you’re breaking ionic bonds and creating new chemical interactions with water. It’s chemistry in action.
Recommendations and Tips for Practical Observation
- Try evaporating saltwater: Watch the salt reform unchanged. This shows the physical aspect of solubility.
- Measure conductivity: Dissolved ions conduct electricity, proving ionic dissociation happens—a chemical change indicator.
- Experiment with other solutes: Sugar dissolves physically without ionization, showing solubility can purely be physical.
Each of these simple experiments can clarify the intricacies of solubility and dissolution.
In Summary
Solubility is primarily a physical property because the substance can usually be recovered unchanged after dissolution. However, the process involves some chemical interactions, especially with metallic ions forming complexes with water.
Dissolving salt in water is a chemical change. The salt’s ionic bonds break into separate ions, which then interact chemically with water molecules. No new substance forms, but the change in bonding qualifies as chemical.
Understanding this subtle dance between physical and chemical changes helps demystify everyday phenomena and deepens appreciation for the chemistry hidden in plain sight.
So, the next time your salty snack melts into a drink, remember: beneath the surface lies a fascinating molecular story of broken bonds and new interactions—not just a simple mix-up.
Is solubility a chemical or physical property?
Solubility is often viewed as a physical property since the substance can usually be recovered unchanged. However, certain interactions, like metal ions forming complexes with water, show chemical aspects.
Why can solubility be considered both physical and chemical?
At a basic level, dissolving does not change the substance chemically, so it’s physical. But in some cases, ions form new bonds with water molecules, which is a chemical process.
Is dissolving salt in water a physical or chemical change?
It is considered a chemical change because the salt’s ionic bonds break. Sodium chloride separates into sodium and chloride ions when dissolved.
Can the salt be recovered after dissolving in water?
Yes. Since the ions remain chemically the same, salt can be extracted from the solution without changing its composition.
What happens to the chemical bonds when salt dissolves in water?
The ionic bonds between sodium and chloride ions break apart. These ions then exist separately in the water, illustrating a chemical change at the ionic level.





Leave a Comment