Molecular Orbital Theory Explained
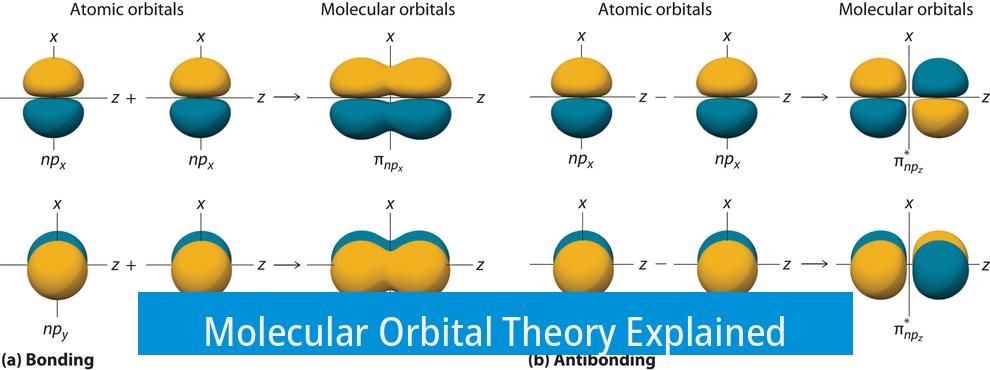
Molecular Orbital Theory describes how atomic orbitals combine to form new orbitals that extend over two or more atoms, called molecular orbitals. These orbitals govern the behavior of electrons in molecules and explain bonding, magnetism, and electronic properties.
Concept of Molecular Orbitals
Molecular orbitals arise when atomic orbitals from bonded atoms overlap. For example, valence orbitals such as 3px, 3py, and 3pz from one atom mix with corresponding orbitals from another atom. This overlap forms molecular orbitals that are shared by both atoms in the molecule. Electrons occupy these molecular orbitals, which can be bonding, antibonding, or non-bonding in nature.
Applications and Importance
- Molecular orbital theory provides insight into chemical bonding beyond simple Lewis structures.
- It explains magnetic behavior by considering unpaired electrons in molecular orbitals.
- The theory helps predict electronic and spectroscopic properties of molecules.
- Understanding orbitals helps elucidate reaction mechanisms, such as nucleophilic substitution (SN2), where an incoming molecule attacks an antibonding orbital of a target molecule.
Learning Molecular Orbital Theory
Grasping molecular orbital theory often requires multiple readings and visual aids. Visualizations of molecular orbitals clarify how atomic orbitals combine and how electron density distributes in molecules.
For beginners and students, multimedia resources like videos can enhance understanding:
Reaction Mechanisms and Orbitals
Molecular orbitals are crucial in explaining how chemical reactions occur. For example, in SN2 reactions, a nucleophile attacks the antibonding molecular orbital of a substrate molecule. This interaction raises the energy of the molecule and breaks the bond. Such orbital-based explanations add clarity to the underlying steps of many reaction mechanisms.
Recommended Reading
More detailed textual information is available in online resources, which cover molecular orbital theory comprehensively:
Key Takeaways
- Molecular orbitals form when atomic orbitals combine across bonded atoms.
- They explain bonding, magnetism, and electronic properties of molecules.
- A deep understanding requires multiple reviews and visual materials.
- Orbital theory offers valuable insight into reaction mechanisms like SN2.
- Reliable video and text resources support learning at different levels.
What is the basic idea behind molecular orbitals?
Molecular orbitals form when atomic orbitals from atoms combine. For example, valence electrons in 3px, 3py, and 3pz orbitals from one atom mesh with those of another to create shared molecular orbitals.
How does molecular orbital theory explain chemical reactions like SN2?
In SN2 reactions, a molecule attacks another’s antibonding orbital. This attack fills the antibonding area, raises the molecule’s energy, and breaks bonds, allowing the reaction to proceed.
Why is it recommended to read about molecular orbital theory multiple times?
Molecular orbital theory has complex ideas that need repeated reading—often three or four times—to grasp the main points. Visual aids and videos also help deepen understanding.
What useful information can molecular orbitals provide about molecules?
Molecular orbitals reveal bonding patterns, magnetic properties, and electronic characteristics. They help explain how atoms share electrons and how this affects the molecule’s behavior.
Where can I find reliable resources to study molecular orbital theory?
Online textbooks like Chem LibreTexts offer detailed explanations. Videos such as those on YouTube provide visual and college-level insights but may require multiple viewings to fully understand.




Leave a Comment