Organic Chemistry Functional Groups: A Detailed Overview
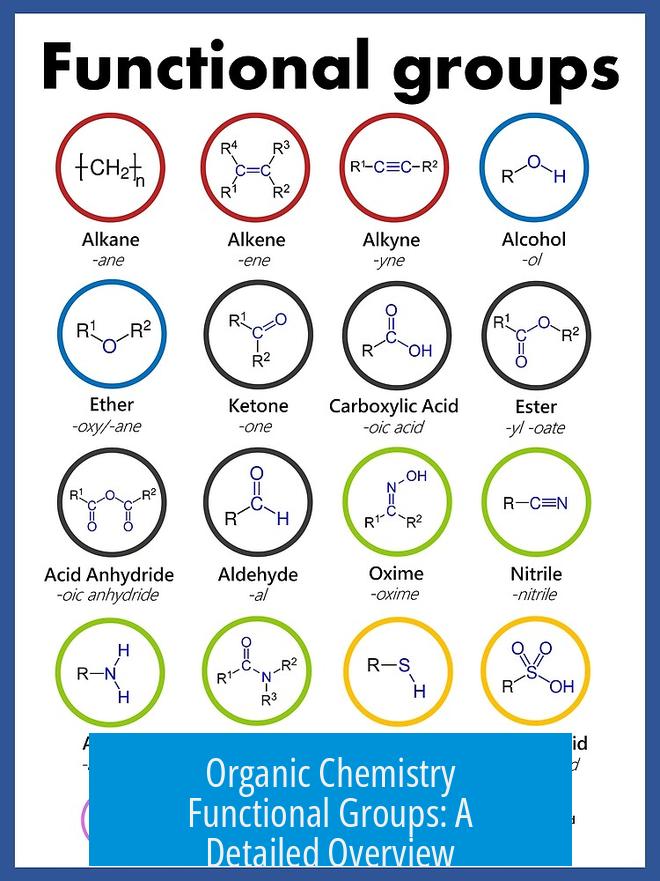
Organic chemistry functional groups are specific groups of atoms within molecules that determine their chemical behavior. These groups influence reactivity, physical properties, and nomenclature of organic compounds. Understanding them is essential to predict molecular interactions and design synthesis routes in chemistry.
Alcohols
Alcohols contain a hydroxyl (-OH) group attached to a carbon atom. They vary from simple methanol to complex structures. Methanol is toxic and can cause blindness, unlike ethanol, which is commonly consumed.
- General formula: R–OH
- Common types: methanol, ethanol, propanol
- Applications: solvents, antiseptics, fuel
Proper handling is critical. Methanol consumption is dangerous and does not represent a healthy lifestyle choice for leisure.
Sulfur-Containing Functional Groups
Sulfur atoms appear in diverse functional groups, each exhibiting unique properties and reactivity. Precise spelling is vital: “sulfur” is preferred over “sulphur” to comply with IUPAC nomenclature standards.
| Functional Group | Structure Example | Note |
|---|---|---|
| Thioether (Sulfide) | R–S–R’ | Analogous to ether but with sulfur |
| Sulfoxide | R–S(=O)–R’ | Sulfur bonded to oxygen |
| Sulfenic Acid | R–S–OH | Rare, highly reactive |
| Sulfone | R–S(=O)2–R’ | Oxidized sulfur group |
| Sultone | Cyclic sulfonate ester | Ring structure incorporating sulfonic group |
Inclusion of these groups is crucial for comprehensive coverage of sulfur chemistry. Charting these alongside phosphorus groups enhances clarity and utility.
Phosphorus-Containing Functional Groups
Phosphorus groups exhibit considerable chemical diversity. Important examples include:
- Phosphonates (–PO(OH)2 bound to carbon)
- Phosphonic Acid (–PO3H2)
- Phosphenic Acid (hypothetical and less common)
These groups play significant roles in biochemistry and materials science. Presenting them with sulfur analogs facilitates understanding of parallel chemistries.
Halides
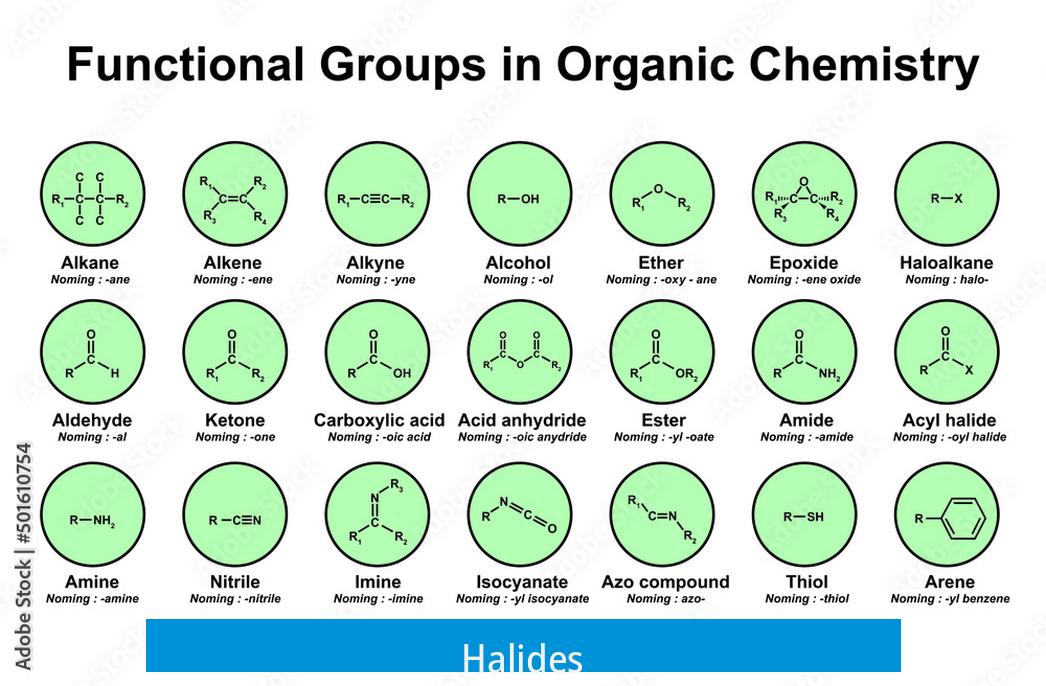
Halides feature halogen atoms (F, Cl, Br, I) bonded to carbon. Naming conventions can vary:
- As prefixes: chloro-, bromo-, fluoro-
- Or suffixes: e.g., “ethyl chloride” instead of “chloroethane”
This flexibility in naming reflects historical and practical usage. Accurate naming emphasizes functional group identity.
Amines and Amides
Amines contain an amino group (–NH2), whereas amides link an amino group to a carbonyl (C=O). Example accuracy matters:
- “Methanamine” is often confused with hexamethylenetetramine; actual simple amine is ethylamine.
- Amines are generally more reactive than amides due to resonance stabilization in amides.
- Reactions that occur with amines do not always extend to amides unless activated.
This distinction impacts synthetic applications, such as functionalization of biopolymers like spider silk.
Alkenes
Alkenes possess carbon-carbon double bonds with formula CnH2n. The IUPAC preferred name for the simplest alkene is “ethene” rather than “ethylene,” though the latter remains widely used.
- General formula: C=C
- Common example: ethene (ethylene)
- Characteristic reactivity: electrophilic addition
Esters
Esters form from an acid and alcohol, featuring R–COO–R’ groups. Correct nomenclature is critical:
- The suffix “-oate” refers to the conjugate base; the alkyl group on the oxygen is named first.
- Formally, esters are named as alkyl carboxylates, e.g., methyl acetate (CH3COOCH3).
- Structural precision avoids misinterpretation; an incorrect extra carbon on the alkyl side is inaccurate.
Other Functional Groups
Additional groups enrich organic chemistry’s scope and include:
- Enones (α,β-unsaturated ketones)
- Acetals and hemiacetals (derived from aldehydes/ketones)
- Oximes (C=NOH), N-oxides, and peroxides
Including these groups improves the depth of understanding and relevance to biochemical and synthetic contexts.
Presentation and Educational Resources
Organizing functional groups by acidity (pKa values) would dramatically enhance their practical value. Color-coded charts help but require clear legends.
Open-access infographics, including SVG and PDF formats, provide flexible educational tools. One biochemistry student’s blog offers updated files that deepen learner engagement.
Summary of Key Points
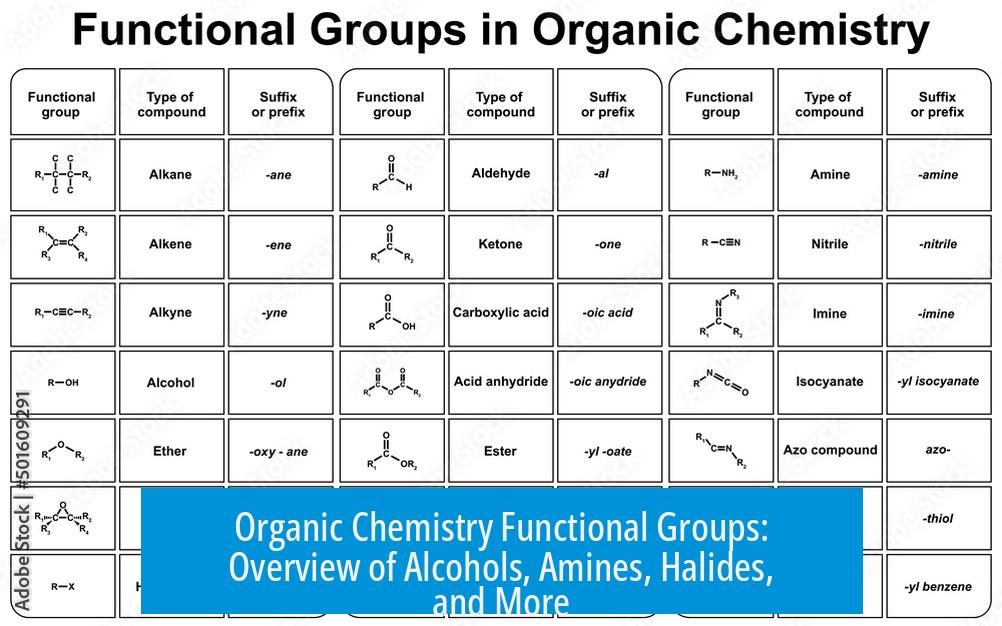
- Sulfur functional groups require standard spelling and expanded coverage (thioether to sultone).
- Phosphorus groups like phosphonates and phosphonic acid should be included alongside sulfur analogs.
- Halide nomenclature can use prefixes or suffixes; both are accepted.
- Amines and amides differ in reactivity; amine examples must be accurate.
- Alkene IUPAC names favor “ethene,” not “ethylene.”
- Esters require precise naming and structural accuracy.
- Additional groups (enone, acetal, oxime) enrich the functional group set.
- Charts benefit greatly from pKa ordering and clear color coding.
- SVG and PDF files enhance accessibility and teaching effectiveness.




Leave a Comment