Understanding the Periodic Table: Styles and Organization
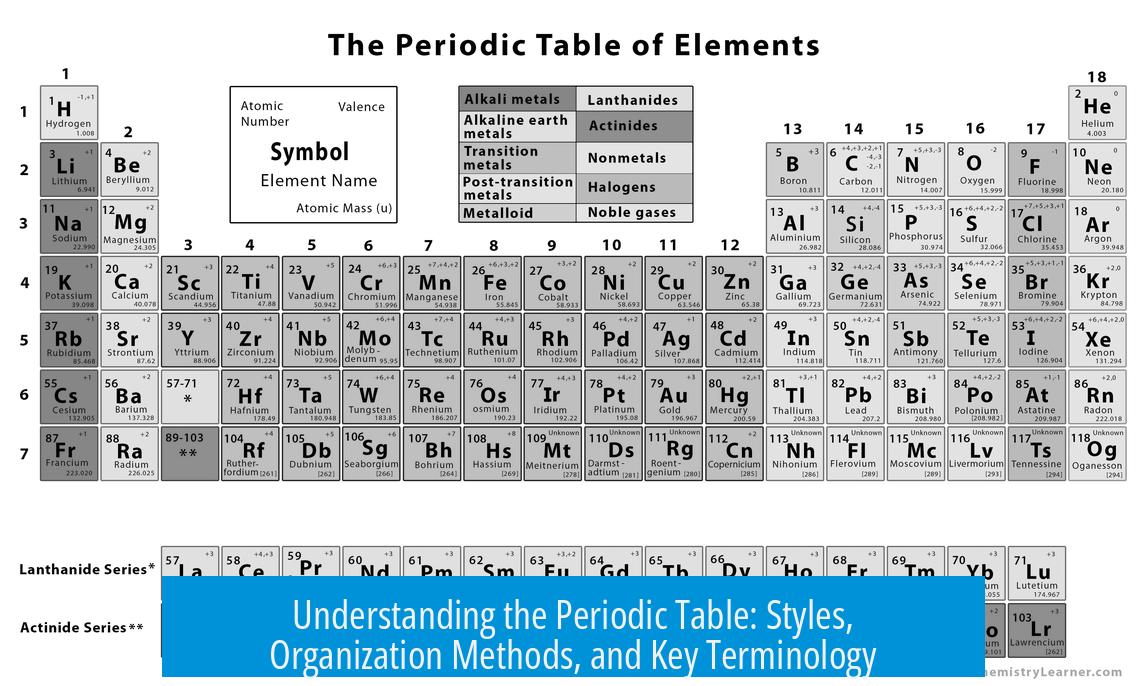
The Periodic Table organizes chemical elements in a structured way, typically by increasing atomic number and by element properties such as valency. Various styles and layouts of the table exist, reflecting historical and regional preferences. One notable style is the short form Russian table, which closely resembles Mendeleev’s original design.
Styles of the Periodic Table
- Old styles of the table, such as those from before the mid-1960s, often display elements up to atomic number 102. This suggests a timeframe after 1957 but before modern expansions.
- The short form Russian style table organizes elements differently, focusing on valency, making it distinct from the standard long form commonly used worldwide.
- This style feels nostalgic to many, as it evokes memories of childhood classroom posters or laboratory walls from the 1970s.
Organization Method
This alternative table style sorts elements primarily by their valency, the number of bonds an element typically forms. Although unusual at first, it proves practical once the user becomes familiar with it.
Such tables may also include details beyond atomic number, such as neutrons along with atomic mass, providing a more comprehensive elemental overview.
Terminology and Classification Notes
- The naming convention for this valency-based ordering differs; it does not follow the standard IUPAC naming scheme.
- There remains debate about classification; for example, actinides are sometimes referred to as rare earth elements, though traditionally rare earths imply lanthanides only.
Nostalgia and Usage
Many collectors cherish these older periodic tables as memorabilia. They find aesthetic and historical value in owning posters or framed tables, with some even assembling puzzles of them.
These tables hold personal connections, often linked to family, education, or historic museums, underscoring their cultural as well as scientific significance.
| Feature | Standard Table | Russian Short Form Table |
|---|---|---|
| Organization Basis | Atomic Number, Groups, Periods | Valency Focused |
| Included Elements | Up to Current Known Elements (118+) | Up to Element 102 |
| Additional Data | Atomic Number, Mass | Includes Neutron Count & Mass |
| Classification Approach | Lanthanides, Actinides as Separate Blocks | Questioned Actinide-Rare Earth Grouping |
Key Takeaways
- The periodic table can be arranged in various formats; the Russian short form emphasizes valency and includes neutron numbers.
- Old styles of the table reflect historical understanding and include fewer elements than modern tables.
- Classification of certain element groups, like actinides, is not universally agreed upon.
- Many people treasure past versions for their educational value and nostalgic appeal.
What is unique about the short form Russian style periodic table?
It closely resembles Mendeleev’s original design. The table organizes elements by valency, which may seem unusual but is practical once understood.
Does this style of the periodic table include neutrons with the elements?
Yes, this version includes neutrons alongside atomic mass, providing a more complete picture of each element’s atomic structure.
Are actinides classified as rare earth elements in this table?
Actinides are sometimes grouped with rare earth elements. However, they have distinct properties separating them from lanthanides often called rare earths.
How does organizing elements by valency help users?
Grouping by valency highlights chemical bonding trends. It may appear odd at first but aids in predicting element reactions and behavior.
Why is there nostalgia around this periodic table style?
Many remember seeing this table in schools, labs, or museums during the mid-20th century. Personal collections and puzzles also bring sentimental value.



Leave a Comment