How to Determine the Number of Valence Electrons in an Element
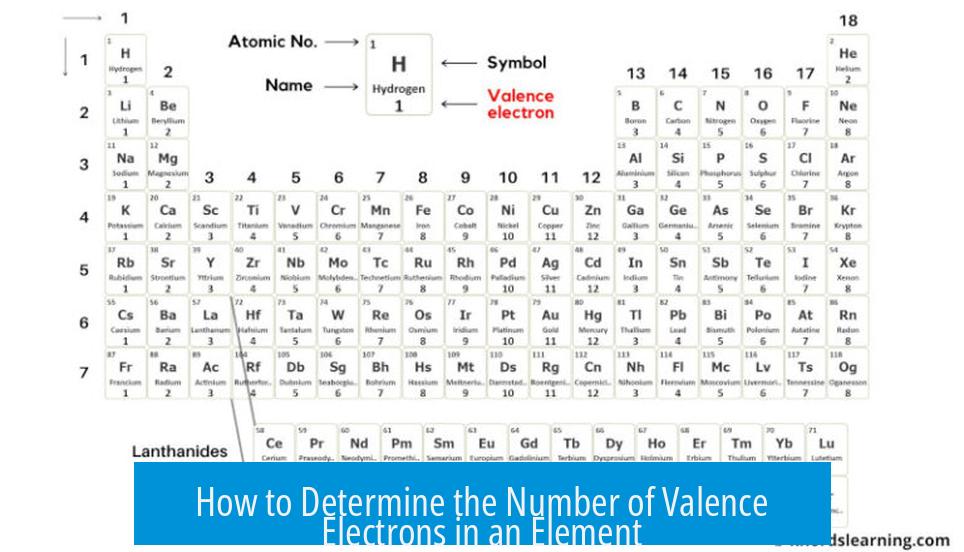
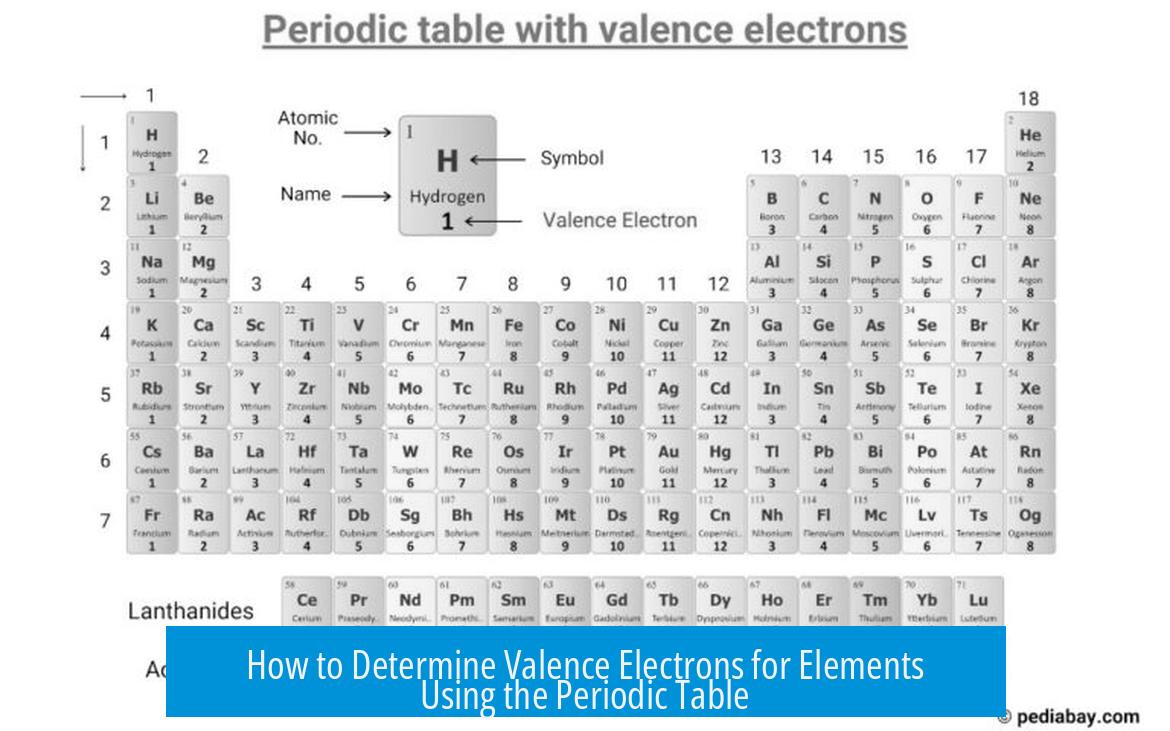
The number of valence electrons in an element equals the main group (or column) number of that element in the periodic table, specifically for main group elements (s- and p-block). This provides a quick and reliable method to identify valence electrons.
Using the Periodic Table Columns
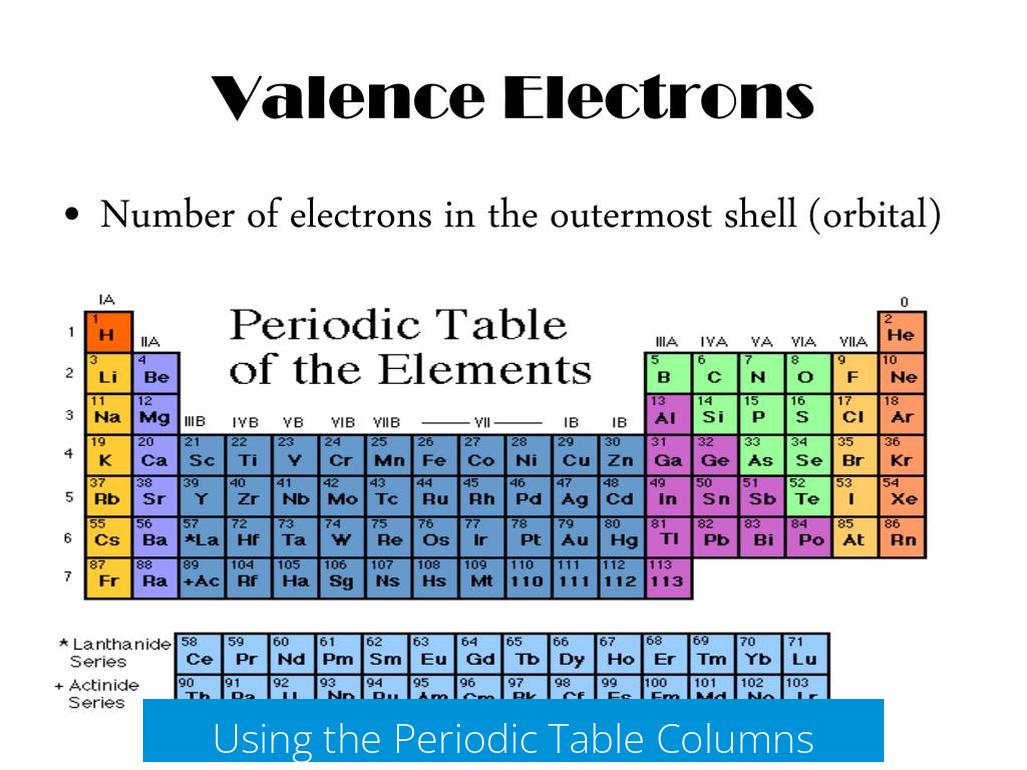
To find valence electrons, first locate the element on the periodic table. Identify its column, also called the main group number. This number directly indicates how many valence electrons the element has.
- Elements in Group 1 have 1 valence electron (e.g., Hydrogen – H).
- Elements in Group 14 (or 4 in older system) have 4 valence electrons (e.g., Carbon – C).
- Elements in Group 17 (or 7) have 7 valence electrons (e.g., Chlorine – Cl).
Specifics on Main Groups and Numbering
Main groups include Groups 1, 2, and 13 to 18 in the modern numbering system. The older system sometimes labels Groups 13 to 18 as 3 to 8. Both systems help determine valence electrons for s- and p-block elements.
Limitations of This Method
This approach applies primarily to main group elements—those in s- and p-blocks of the periodic table. It does not work well for transition metals or inner transition metals (d- and f-block elements). Elements like Iron (Fe) or Gold (Au) require a more detailed electron configuration analysis.
Summary Table: Valence Electrons in Main Group Elements
| Group Number | Valence Electrons | Example Element | Valence Electrons |
|---|---|---|---|
| 1 | 1 | Hydrogen (H) | 1 |
| 2 | 2 | Magnesium (Mg) | 2 |
| 13 / 3 | 3 | Aluminum (Al) | 3 |
| 14 / 4 | 4 | Carbon (C) | 4 |
| 15 / 5 | 5 | Nitrogen (N) | 5 |
| 16 / 6 | 6 | Oxygen (O) | 6 |
| 17 / 7 | 7 | Chlorine (Cl) | 7 |
| 18 / 8 | 8 | Neon (Ne) | 8 |
Key Takeaways
- Valence electrons equal the main group number for s- and p-block elements.
- Locate the element’s column in the periodic table to find valence electrons quickly.
- This method applies only to main group elements, not transition metals.
- Group numbering may appear as 1-2 and 13-18 in modern tables.




Leave a Comment