Understanding SO42- Ion
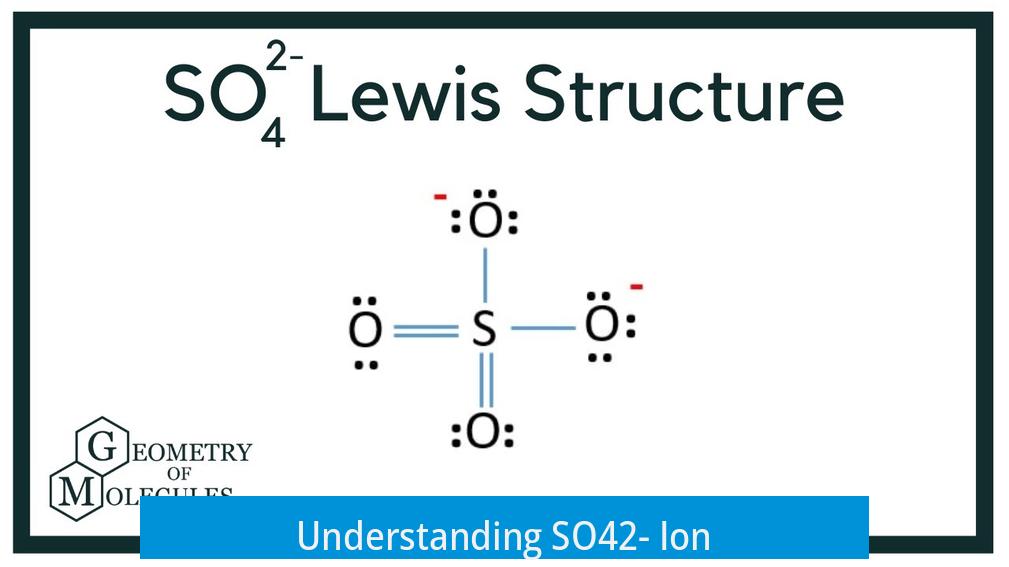
The SO42- ion is a polyatomic ion containing sulfur and oxygen, where all sulfur-oxygen (S–O) bonds are covalent. This ion carries a charge of -2 due to extra electrons distributed over the oxygen atoms. It plays a major role in various chemical compounds.
Nature and Structure of SO42-
SO42- consists of one sulfur atom centrally bonded to four oxygen atoms. These bonds form through covalent interactions. The electrons are shared between sulfur and oxygen atoms, creating a stable molecular shape. Resonance structures allow electron density to be delocalized across the molecule. This stabilizes the ion and accounts for bond length uniformity.
Bonding Characteristics
While the S–O bonds inside the ion are covalent, the entire sulfate ion can interact ionically with other species. When combined with metal cations such as magnesium, it forms ionic compounds. For example, magnesium sulfate (MgSO4) features Mg2+ ions bonded ionically to SO42- ions. This ionic bonding arises from the electrostatic attraction between the positively charged cations and the negatively charged sulfate ion.
Example of Ionic Bonding with SO42-

- Magnesium sulfate (MgSO4): Mg2+ cations bond ionically to sulfate anions.
- This compound dissolves easily in water, dissociating into Mg2+ and SO42- ions.
- Applications include use in medicine, agriculture, and industrial processes.
Summary
- SO42- is a polyatomic ion with covalent S–O bonds.
- The ion carries a -2 charge distributed over oxygen atoms.
- It forms ionic bonds with metal cations, such as Mg2+, resulting in salts like MgSO4.
- This dual bonding nature influences the chemical behavior and application of sulfate compounds.
Unlocking the Mystery of SO42−: More Than Just a Sulfate Ion
Let’s cut to the chase: SO42− is a polyatomic ion with all sulfur-oxygen bonds formed covalently, but the ion itself bonds ionically to metals like magnesium, creating compounds such as MgSO4. Simple, right? But stick around—this isn’t your typical chemistry class snippet. There’s a story behind those atoms holding hands, and it shapes the world around you.
First things first, what exactly is SO42−? Known as the sulfate ion, this chemical group features one sulfur atom centrally located and bonded to four oxygen atoms. The “2−” charges mean the ion carries twice the negative charge. This combination creates a highly reactive player in chemistry, environmental science, and even your daily life.
What Does It Mean That SO42− Is a Polyatomic Ion?
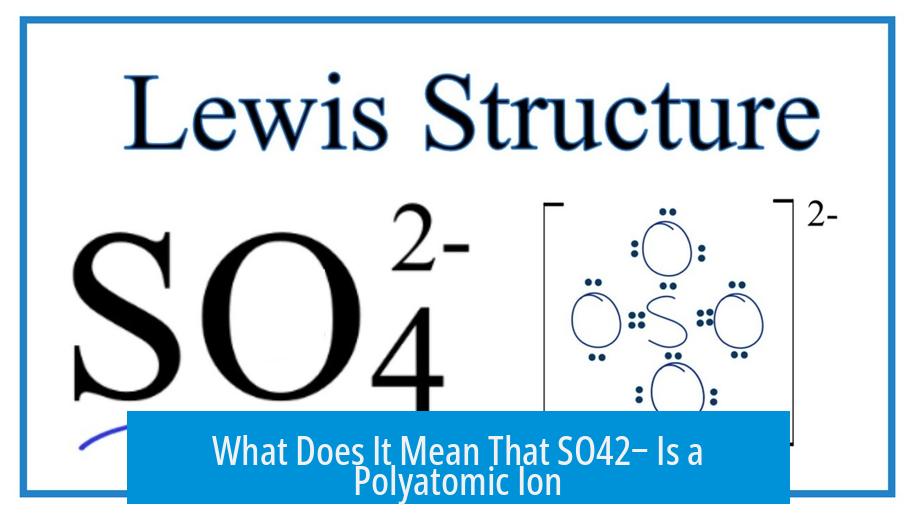
A polyatomic ion is a cluster of atoms that travel together with a net electrical charge. Unlike your lone atoms like Na+ or Cl−, polyatomic ions are like tiny molecular families. SO42− is one such family—one sulfur “parent” and four oxygen “kids” tightly bonded.
Here, the S–O bonds are all covalent. That means they share electrons rather than give or take. So, within the ion, electrons get a “shared custody” arrangement among sulfur and oxygen. This covalent bonding is key to the ion’s stability and explains why the sulfate ion can exist comfortably in water and in many compounds.
Interestingly, while the bonds holding sulfur and oxygen together are covalent, the overall ion behaves like a charged entity ready to mingle with positively charged atoms, or cations.
But Wait—How Does SO42− Interact With Other Elements?
This is where things get juicy. The sulfate ion itself is negatively charged. So, in chemistry world where opposites attract, SO42− seeks out positive partners—cations— to form neutral, solid compounds.
Take magnesium sulfate, MgSO4, as an example. Mg2+ is the positively charged magnesium ion, which bonds ionically with the sulfate ion. Ionic bonding here means that Mg2+ and SO42− stick together by electrostatic forces, not by sharing electrons like the sulfur and oxygen do inside SO42−.
In simpler terms, sulfur and oxygen are holding hands in a tight covalent circle. This entire negatively charged entity then pairs with a positively charged partner like magnesium, forming ionic compounds often used in medicine and chemistry labs.
Why Should You Care About SO42− and Its Bonding Behavior?
Sulfates are everywhere. Dead serious. They pop up in your bath salts (hello, Epsom salts), fertilizers, even water supplies. Understanding SO42− bonding helps explain everything from why those bath salts dissolve so well to why sulfate levels affect environmental water quality.
Let’s talk real-world impact. Magnesium sulfate (MgSO4), often known as Epsom salt, leverages this ionic bonding to be highly soluble in water. The ionic bond with Mg2+ means the compound breaks apart easily, releasing sulfate ions that can help soothe sore muscles. Think of it as anthropomorphizing ions, but with good reason—the way these ions interact affects everything from health to agriculture.
Breaking Down the Science: Bonding at Play
- Within SO42−: All S–O bonds are covalent. Electrons are shared uniformly, giving the ion its rigid, structured identity.
- Between SO42− and cations: Bonds are ionic, relying on the electrostatic attraction between SO42−’s negative charge and the positive charge of metal ions like magnesium’s Mg2+.
This hybrid bonding nature lends SO42− unique properties. For one, it’s stable as an ionic compound but maintains strong internal covalent links. This duality helps chemists predict how compounds behave during reactions, dissolutions, and biological processes.
How Do These Concepts Help You Vendor or Student?
If you’re a student, knowing the different bonding types inside and outside the SO42− ion helps ace those chemistry tests. And for vendors or manufacturers dealing with sulfate compounds, understanding this makes product formulation smarter. For example, tweaking the cation part changes solubility and reactivity.
What happens if you switch magnesium for calcium? You get CaSO4, which is slightly less soluble than MgSO4, affecting industrial processes such as plaster production. Thing is, the sulfate ion consistently brings the same covalent bonding inside but builds different compound properties depending on its ionic buddy.
Final Thoughts: The Duo That Makes SO42− Stand Out
In the grand theater of chemistry, SO42− plays a double role. It’s a close-knit covalent family internally but a charged, social entity externally, bonding ionically with metals. This dual personality enables a vast range of practical uses and makes SO42− a prime example of chemistry’s complexity—wrapped in a simple little ion.
So next time you see MgSO4 or even just hear “sulfate,” remember it’s not just some random ion. It’s a cooperative dance of bonds shaping industries, health, and the environment. And honestly, isn’t that way cooler than your average ion?
What type of bonds exist within the SO4^(2-) ion?
All the sulfur-oxygen (SO) bonds inside the SO4^(2-) ion are covalent. This means electrons are shared between sulfur and oxygen atoms within the ion.
How does SO4^(2-) bond with other elements?
SO4^(2-) forms ionic bonds with cations like magnesium. For example, in MgSO4, the SO4^(2-) ion pairs ionically with Mg²⁺ ions.
Is SO4^(2-) a single atom or a group of atoms?
SO4^(2-) is a polyatomic ion, which means it consists of multiple atoms bonded together covalently to form one charged ion.
Why is SO4^(2-) considered polyatomic?
Because it contains multiple atoms (one sulfur and four oxygens) bonded together, creating a single ion with an overall 2- charge.
Can SO4^(2-) form covalent bonds with cations?
No, SO4^(2-) forms ionic bonds with cations. The covalent bonds occur only inside the SO4^(2-) ion among sulfur and oxygen atoms.


Leave a Comment