Synthetic Opal: How to Make at Home?
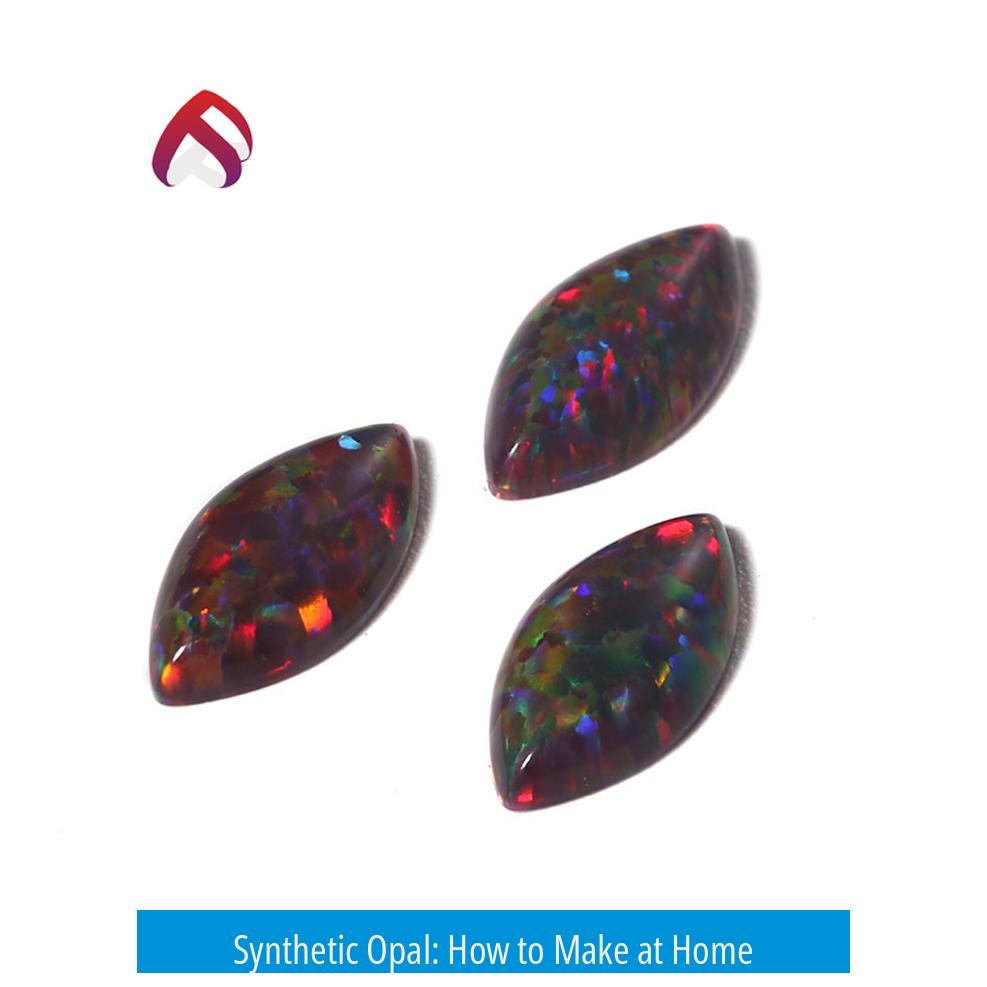
Synthetic opal can be made at home by carefully following a chemical synthesis process using tetraethyl orthosilicate (TEOS), ammonium hydroxide diluted with ethanol, and precise temperature control. This process involves the formation and self-assembly of silica nanospheres, mimicking the natural opal structure.
Chemicals and Ingredients Required

- Tetraethyl orthosilicate (TEOS): The key silica source essential for opal synthesis. Supplies silicon atoms needed to form silica spheres.
- Ammonium hydroxide: Acts as a catalyst. It is diluted with ethanol to control the reaction’s pH and kinetics.
- Ethanol: Used as a solvent and diluent for ammonium hydroxide and TEOS mixtures.
- Additional Hardening Agents: Sometimes incorporated to improve the synthetic opal’s durability.
- Reagent-grade chemicals: High-purity solvents and reagents ensure quality results and minimize impurities.
Essential Equipment and Safety Setup

- Hotplate with Magnetic Stirrer: Maintains constant heating (~65°C) and homogeneous stirring for controlled reaction.
- Laboratory Glassware: Flasks, beakers, and stir bars designed to handle chemical reagents safely.
- Fume Hood: Crucial to avoid inhalation of toxic ammonia vapors and ethanol fumes.
- Personal Protective Equipment: Gloves, goggles, and lab coat to prevent exposure to hazardous chemicals.
- Small Home Lab Setup: Possible but requires careful preparation and strict adherence to safety protocols.
Step-by-Step Procedure

- Prepare Ammonium Hydroxide Solution: Dilute ammonium hydroxide with ethanol, then heat mixture to approximately 65°C while stirring.
- Mix TEOS and Ethanol: In a separate flask, combine TEOS with ethanol and any additives intended for hardening.
- Combine Solutions: Add the TEOS-ethanol mixture slowly to the heated ammonium hydroxide-ethanol solution to start silica condensation.
- Stir Reaction Mixture: Maintain stirring and temperature control for about 8 hours to allow silica nanospheres to form and organize.
- Let the Solution Age: Initially, only a fine white powder appears. After several days and potentially up to two weeks, opal-like material forms at the bottom of the container, layering and developing play-of-color.
- Optional Mold Growth: The synthetic opal slurry can be poured into molds to shape the resulting gem.
Chemical Composition and Structure of Opal
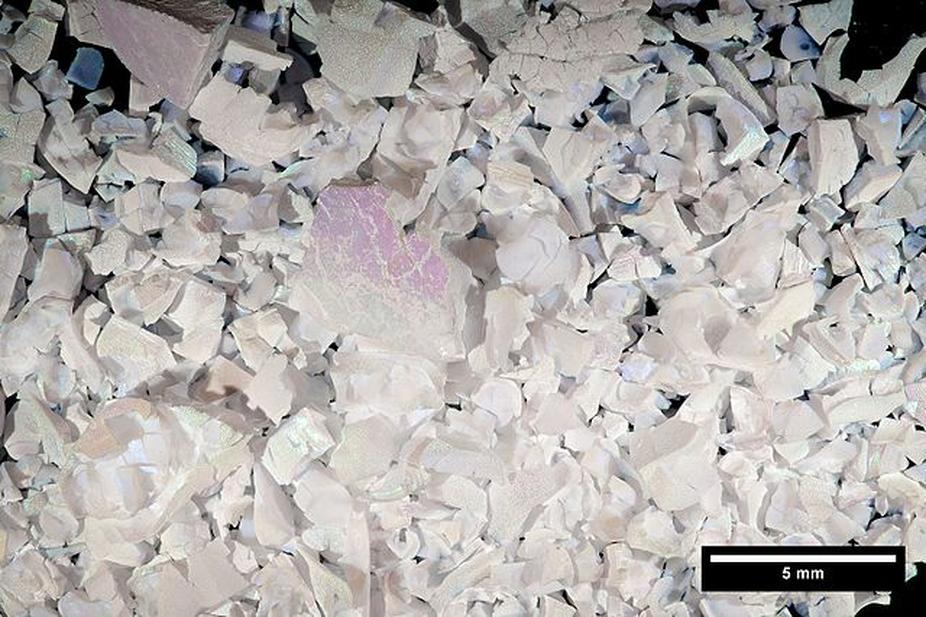
Opals are composites of spherical silica nanoparticles assembled into a highly ordered lattice. This periodic arrangement causes diffraction of light, producing the opal’s iridescence, or play-of-color. Synthetic opals replicate this nanosphere structure by spontaneously organizing during the sol-gel reaction.
Lab-synthesized opals have nearly identical chemistry and structure to natural opals, making them visually and physically very similar. The main difference often lies in their uniformity: synthetic opals tend to have consistently sized spheres and a more even color display.
Safety Considerations

- Ammonium hydroxide releases harmful ammonia gas, which irritates respiratory pathways. Proper ventilation and a fume hood are essential.
- Use reagent-grade chemicals to minimize contamination and hazards.
- Wear appropriate PPE to avoid skin and eye contact with chemicals.
- Heating organic solvents like ethanol requires careful monitoring to prevent fire hazards.
Types of Synthetic Opals
Synthetic opals can be crafted in various forms, including black opal, white opal, crystal opal, and fire opal analogs. Each variety depends on slight changes in synthesis conditions and additives, influencing color brightness and pattern. The fundamental principle remains constant: growth of uniform silica spheres arranged in a lattice.
Additional Resources and Community Insights
Detailed recreations and discussions about home synthesis appear in specialized online forums. Resources such as how to grow opals guides provide accessible entry points for beginners. Engaging with experimental communities helps troubleshoot synthesis challenges, especially in later stages like filling opal structures with silica gel.
Key Takeaways
- Synthetic opal is made by forming and organizing silica nanospheres from TEOS via ammonium hydroxide-catalyzed sol-gel reactions.
- Careful temperature control (~65°C) and extended stirring (about 8 hours) are necessary.
- The process takes days to weeks for visible opal formation.
- Using proper lab equipment and strict safety measures, including a fume hood, is essential due to toxic ammonia and flammable ethanol.
- Synthetic opals have chemical and structural equivalence with natural opals but exhibit more uniformity.
- Variations exist to mimic different opal types, including black, white, and fire opals.
Synthetic Opal: How to Make at Home?
Yes, you can make synthetic opals at home—but it’s no weekend craft project. It requires specific chemicals, careful heating, lots of patience, and some serious safety gear. These lab-made beauties are almost twins of natural opals, mimicking the dazzling color show thanks to their precisely arranged silica nanospheres. But how does one weave such magic from chemicals to shiny gems? Let’s dive in.
First, a quick shout-out to the queen of the show: tetraethyl orthosilicate (TEOS). This is the superstar ingredient for the silica structure underlying synthetic opals. You won’t find this at your average home store, so ordering it online is necessary. TEOS acts as a silicon source, essential to build the tiny spheres that give opals their characteristic glow.
Alongside TEOS, you need ammonium hydroxide (a strong base that can clear those nostrils—but in the bad way) and ethanol. Mixing these carefully sets the stage for the silica spheres to form. The process starts by diluting ammonium hydroxide with ethanol, then gently heating the mix to about 65°C on a hotplate or magnetic stirrer. We’re talking lab-level precision here.
Safety First (Before You Feel Like a Gem Scientist)
Before you get excited about shiny new opals, hold your horses. Ammonium hydroxide fumes can be nasty, and the entire procedure involves chemicals that must be handled with proper lab ware, safety goggles, gloves, and ideally inside a good fume hood. DIY does not mean “eyeball it and hope for the best.” Protect yourself.
The Step-by-Step Alchemy Behind Synthetic Opals
The procedure unfolds in stages, each requiring patience. After preparing your ammonium hydroxide and ethanol blend, you combine TEOS with ethanol in a separate flask, adding a few other chemicals that help the formation and hardening of the opal. Then, this new mixture joins the ammonium ethanol solution.
Next is the waiting game—about 8 hours of stirring to encourage the silica particles to start assembling. But don’t expect magic overnight. Early on, you’ll just notice some white powder settling at the bottom of your container. This is your precious silica spheres beginning their slow dance. After around two weeks, you should start to see a quarter-inch layer of synthetic opal forming.
Here’s the kicker: the synthetic opal needs extra fortification. Experimenting with additional hardening agents is a smart move, as raw synthetic opals are often fragile. Finding the right mix can transform your creation from a delicate experiment into a durable gem worthy of display.
How Close Is Synthetic to Natural?
Synthetic opals boast nearly identical chemical structures to their natural counterparts. The main difference? Synthetic opals look more uniform. Natural stones carry the lovely imperfections of the earth, while lab-grown gems have that “too perfect” glow. For most eyeballs, though, synthetic opals pass the authenticity test with flying colors.
Why Bother Making Synthetic Opals at Home?
If you have an obsession with geology or fancy yourself a gemologist, this DIY process offers a satisfying peek into crystal growth science. It’s also a brilliant way to get your hands on opals without the hefty price tag. Plus, controlling the creation process means you can experiment with color variations and hardness to suit your artistic vision or jewelry projects.
Practical Tips for Would-Be Gemmakers
- Order high-purity TEOS and reagent-grade ammonia. Impurities spoil the reaction and may ruin your batch.
- Don’t cut corners on equipment: a hotplate with magnetic stirrer and a reliable fume hood are essential.
- Use proper lab containers, preferably glass, to avoid dangerous reactions with plastics.
- Maintain steady temperature and stirring to encourage uniform silica sphere formation.
- Expect to wait at least two weeks before your synthetic opal emerges. Patience is a virtue here!
- Experiment cautiously with hardening chemicals like silicates or resins after the initial opal forms.
Explore and Learn More
If you want to jump down the rabbit hole further, community discussions and guides like those on Our Pastimes offer valuable insights and ongoing updates. The synthetic opal crafting community is eager to share tips, troubleshoot, and celebrate small wins together.
Curious about replicating this process? Ask questions and look for updated methods from community forums where enthusiasts discuss the tricky parts—especially when “filling the opal with silica” comes up as a sticking point.
In Summary: Is It Worth Trying at Home?
Making synthetic opals at home is challenging but rewarding. It’s a delicate blend of chemistry, patience, and safety awareness. The process takes time and respect for the chemicals involved. With the right setup—TEOS, ammonium hydroxide, ethanol, heating, stirring, and safety gear—you can create a synthetic opal that dazzles. Careful experimentation and community support help turn your glass jar of chemicals into a shimmering slice of geological art.
Ready to give opal making a whirl? Suit up with your lab gear, stock your chemicals, and set your timer for two weeks. Watching synthetic opal grow might just become your new favorite slow-burn hobby.
What chemicals are essential to make synthetic opal at home?
The key chemical is tetraethyl orthosilicate (TEOS). You also need ammonium hydroxide and ethanol. Additional ingredients may be used to harden the final product.
What equipment is needed for home synthesis of opals?
A hotplate with a magnetic stirrer is required for heating and mixing. Proper lab ware and safety equipment, like a fume hood, are necessary for handling chemicals safely.
How long does the synthetic opal formation take?
The stirring reaction runs about 8 hours. Actual opal growth appears over days to weeks. It may take around two weeks to see noticeable opal forming at the bottom of the container.
How does the process of synthetic opal formation work?
- Create tiny silica nanospheres.
- Arrange these spheres in a precise lattice pattern mimicking natural opal structure.
- Allow the structure to set and harden into solid opal.
Are synthetic opals chemically the same as natural opals?
Yes, synthetic opals have identical chemical composition and structure. They often look more uniform but share the same physical properties as natural opals.
Is it safe to make synthetic opals at home?
The process uses hazardous chemicals like ammonium hydroxide. Proper ventilation and lab safety gear are essential to avoid exposure. Without these, it is unsafe to attempt.





Leave a Comment