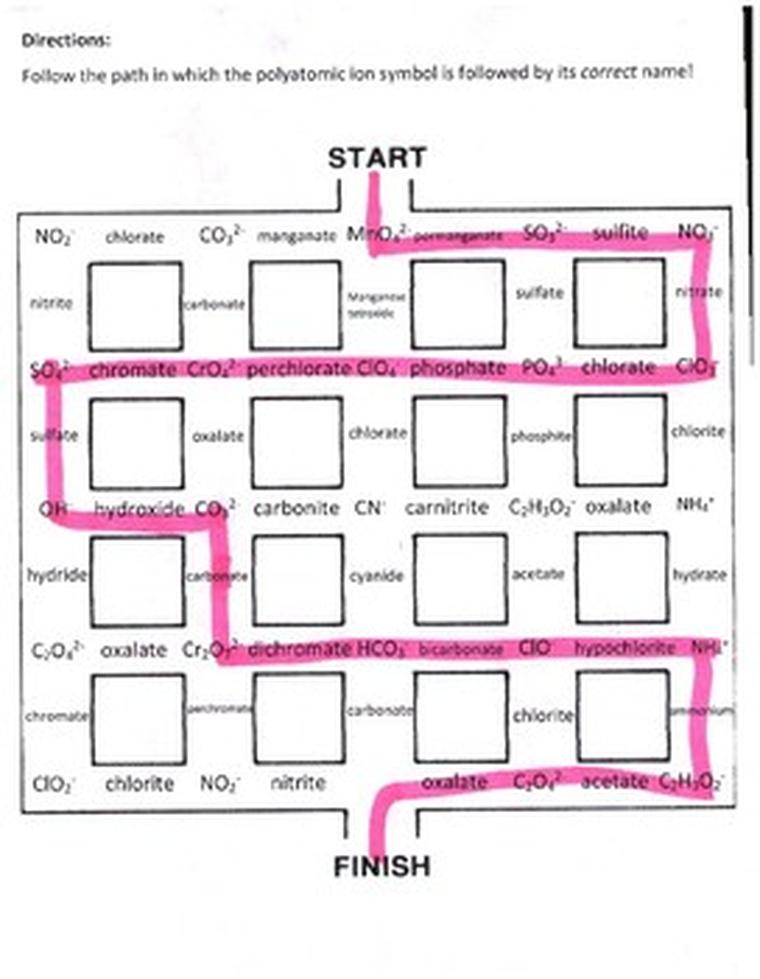
Table for Memorizing Polyatomic Ions Organized by Element, Charge, Prefixes, and Endings
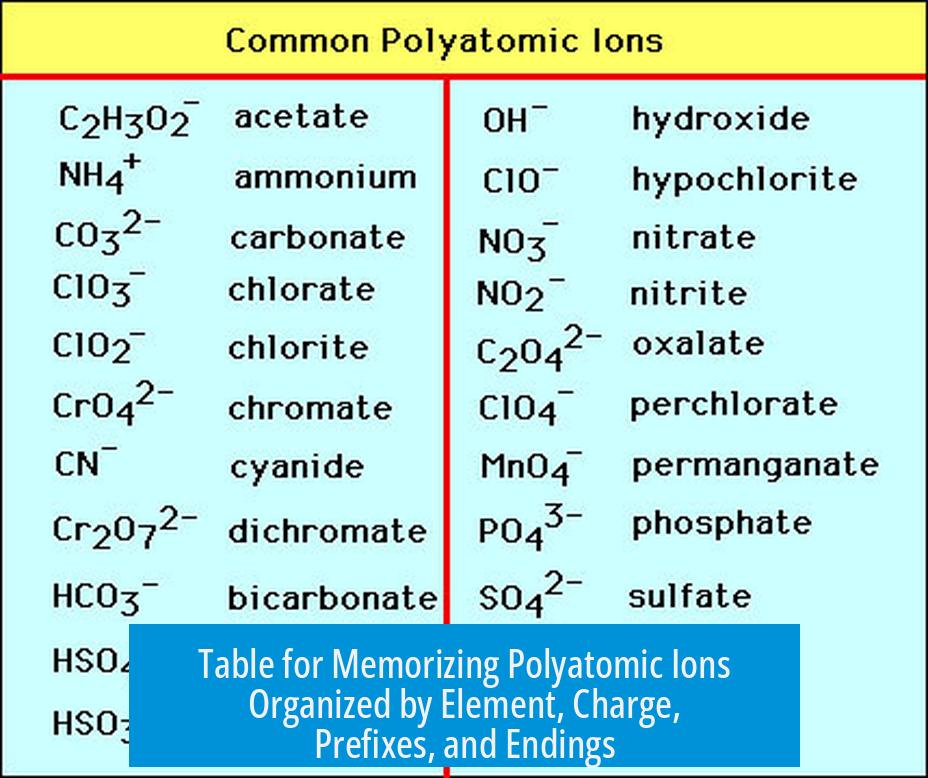
Memorizing polyatomic ions is easier when they are grouped by elements, charges, prefixes, and suffixes. This organized approach not only simplifies recall but also reveals patterns in ion names and structures. Below is a guideline and table to help with this task.
Common Elements in Polyatomic Ions
- Oxygen-containing ions: Such as sulfates, nitrates, phosphates.
- Halogen-based ions: Include chlorates and perchlorates.
- Metalloids and transition metals: Like chromates and manganates.
- Organic ions: Acetates and other carboxylates.
Charge Organization
Polyatomic ions most commonly carry charges of -1, -2, or -3:

| Charge | Example Ions |
|---|---|
| -1 | Nitrate (NO3-), Acetate (C2H3O2-), Chlorate (ClO3-) |
| -2 | Sulfate (SO42-), Carbonate (CO32-), Chromate (CrO42-) |
| -3 | Phosphate (PO43-), Arsenate (AsO43-) |
Prefixes and Endings: Indicators of Oxygen Content and Ion Structure
- Prefix “per-“ indicates one more oxygen than “-ate”. Example: Perchlorate (ClO4-).
- Suffix “-ate” indicates a common or standard oxyanion. Example: Sulfate (SO42-).
- Suffix “-ite” means one less oxygen than “-ate”. Example: Sulfite (SO32-).
- Prefix “hypo-“ indicates one less oxygen than “-ite”. Example: Hypochlorite (ClO-).
- Prefix “pyro-” or “di-“ refers to ions formed by the combination of two units, often seen in disulfate (pyrosulfate, S2O72-) and peroxydisulfate.
Important But Less Common Ions
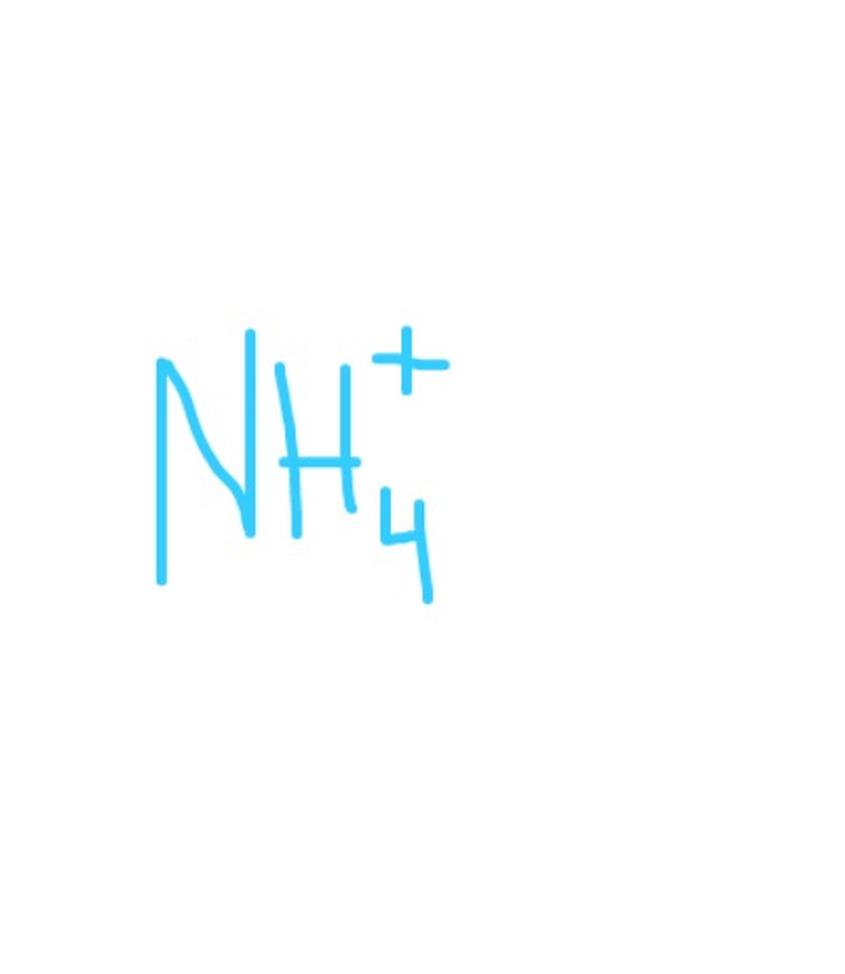
- Disulfate (Pyrosulfate): S2O72-, formed by two sulfate ions minus one oxygen.
- Peroxydisulfate: (S2O82-), contains peroxide linkages, important in oxidation reactions.
Additional Notes on Memorization
Polyatomic ion memorization can be difficult as many ions have subtle differences. For example, forgetting chromate (CrO42-) is a common problem. Grouping ions by element and charge helps in this regard. Different courses have varying requirements for memorization; some do not require full memorization but rather recognition skills.
Example Table for Quick Reference
| Ion | Formula | Charge | Notes |
|---|---|---|---|
| Nitrate | NO3- | -1 | Common oxyanion of nitrogen |
| Chlorate | ClO3- | -1 | Halogen oxyanion |
| Sulfate | SO42- | -2 | Common oxyanion of sulfur |
| Chromate | CrO42- | -2 | Transition metal oxyanion |
| Phosphate | PO43- | -3 | Common oxyanion of phosphorus |
| Acetate | C2H3O2- | -1 | Organic acid ion |
| Disulfate (Pyrosulfate) | S2O72- | -2 | Less common; formed by sulfate ions |
| Peroxydisulfate | S2O82- | -2 | Contains peroxide bond; used as oxidizer |
Summary of Tips
- Group ions by element and charge for easier recall.
- Use prefixes and suffixes as clues to oxygen count and ion type.
- Remember common ions first, then expand to less common ones.
- Practice recalling formula and charge together.
- Recognize that memorization requirements vary across classes.
The Ultimate Table for Memorizing Polyatomic Ions: Organized by Element, Charge, Prefixes, and Endings
Ever had that moment of sheer panic when you realize you need to memorize *all* the polyatomic ions for a chemistry exam? You’re definitely not alone. One brave soul confessed, “Didn’t realize until the day before my first lecture that we had to memorize them all and I cried on my couch several times after forgetting chromate.” If you’re nodding along or cringing at the memory, this post is for you.
 So, what’s the best way to memorize polyatomic ions? The key is to organize them into a table that groups ions by their core element, their charge, and the common prefixes and endings that hint at their structure. This method turns a dreaded chore into a manageable task.
So, what’s the best way to memorize polyatomic ions? The key is to organize them into a table that groups ions by their core element, their charge, and the common prefixes and endings that hint at their structure. This method turns a dreaded chore into a manageable task.
Let’s unravel this together.
Why Memorizing Polyatomic Ions Can Feel Like a Labyrinth
Polyatomic ions are tricky. You’re not just juggling single atoms but clusters of atoms with specific charges. Add to this the frequent use of prefixes like “per-“ and “hypo-“, and suffixes like “-ate” and “-ite”, and you might feel overwhelmed. Not to mention bonus ions like disulfate (pyrosulfate) and peroxydisulfate which don’t always get the spotlight in class.
Many students and professionals find the common ions like sulfate, nitrate, and carbonate straightforward, but the less common ones can throw a wrench in your memory.
A Table That Organizes Polyatomic Ions: Breaking It Down
| Element Central Atom | Ion Example | Charge | Prefix | Ending (Suffix) |
|---|---|---|---|---|
| Chlorine | Perchlorate | -1 | Per- (more oxygen) | -ate |
| Chlorine | Chlorate | -1 | — | -ate |
| Chlorine | Chlorite | -1 | — | -ite (less oxygen) |
| Chlorine | Hypochlorite | -1 | Hypo- (least oxygen) | -ite |
| Sulfur | Disulfate (Pyrosulfate) | -2 | Di- (two sulfur atoms) | -ate |
| Sulfur | Peroxydisulfate | -2 | Peroxy- (O-O bond) | -disulfate |
| Sulfur | Sulfate | -2 | — | -ate |
| Sulfur | Sulfite | -2 | — | -ite |
| Carbon | Acetate | -1 | — | -ate (organic acid) |
This table groups ions primarily by their fundamental element, which helps you identify patterns. For example, sulfur-containing ions often show up as sulfates or sulfites, and prefixes like per- and hypo- typically indicate higher or lower oxygen content, respectively.
Prefixes & Endings: Your Memory’s Best Friend
Here’s a neat mental trick: Rely on prefixes and endings to predict or confirm the identity and charge of an ion. Oxygen-rich ions often come with the prefix “per-“. For example, perchlorate (ClO4−) has more oxygen than chlorate (ClO3−). Conversely, “hypo-“ refers to ions with fewer oxygen atoms, like hypochlorite (ClO−).
And don’t forget suffix changes: “-ate” usually ends ions with more oxygen atoms, while “-ite” marks fewer oxygen atoms; this applies across halogens, sulfur, and nitrogen groups.
Expanding Your Table Beyond Basics
Most memorization guides list typical ions such as sulfate (SO42−), nitrate (NO3−), and acetate (C2H3O2−). But what about the lesser-known ions? For example, the disulfate or pyrosulfate (S2O72−) and the peroxydisulfate (S2O82−) are worth adding to your roster.
Organic acid ions are also underrepresented. Acetate makes the cut often, but there’s a whole zoo of organic polyatomic ions lurking in the background.
Pro tip: If you’re diving into advanced chemistry or environmental science, expand your memorization list to include these, or at least be familiar with their patterns and names. This broadened perspective will turn you from a mere memorizer into a true chemistry connoisseur.
Is It Really Necessary to Memorize All Polyatomic Ions?
This prompts an important question: Do all classes require memorizing every polyatomic ion? The answer is no. One learner shared, “I’ve never had a class that requires anyone to memorise this.” Indeed, the memorization workload depends largely on your course, institution, or exam requirements.
For some, understanding the principles behind naming and charges beats rote memorization. However, having a structured table like the one above still makes your life easier when you *do* encounter tricky ions.
How Can You Use This Table Effectively?
- Study Elements First: Learn the common central atoms (chlorine, sulfur, nitrogen, carbon) and their ion families.
- Focus on Prefixes and Suffixes: Recognize how oxygen counts change ion names.
- Practice Writing Charges: Memorize charge patterns that stay consistent within groups.
- Include Relevant Ions: Add ions like disulfate and peroxydisulfate to your study set if needed.
- Use Visual Aids: Color-code ions or create flashcards referencing the table.
By approaching polyatomic ions as a pattern-recognition puzzle, the task becomes engaging and less intimidating.
Thanks for Sticking Around
Let’s face it: polyatomic ions are a tough cookie. Thanks to dedicated chemists and educators creating handy tables, students can tackle this challenge with a solid roadmap. Remember, if you feel overwhelmed, you’re not alone! Use these tables and tips to turn frustration into mastery.
Want a handy PDF or flashcard deck based on this table? Just ask!
Happy ion hunting!
What is an effective way to organize polyatomic ions for memorization?
Group ions by their central element, then by charge. Use prefixes like hypo-, per- and endings such as -ate, -ite. This approach helps predict ion names and charges.
Why are prefixes and endings important in polyatomic ion names?
Prefixes like “per-” and “hypo-” indicate the number of oxygen atoms. Endings like “-ate” and “-ite” show different oxygen counts. Recognizing these aids recall.
Are there lesser-known polyatomic ions that should be memorized?
Yes. Beyond common ions like acetate, ions such as disulfate (pyrosulfate) and peroxydisulfate are worth learning for a fuller understanding.
Do all chemistry classes require memorizing polyatomic ions?
No. Some courses focus on understanding rather than memorization. Requirements vary by instructor and curriculum.
Can grouping polyatomic ions reduce memorization difficulty?
Yes. Organizing by element and charge provides patterns that simplify recall. Predictable prefixes and endings also help identify and memorize them faster.



Leave a Comment