Theoretical Buffer Capacity Calculation

Theoretical buffer capacity can be calculated using the Henderson-Hasselbalch equation by adjusting acid and base concentrations to achieve a desired pH. However, practical limitations reduce its usefulness, requiring empirical methods for precise buffer preparation.
Using the Henderson-Hasselbalch Equation

The Henderson-Hasselbalch equation relates pH, acid concentration, base concentration, and the acid dissociation constant (pKa).
For a buffer, the pH is given by:
pH = pKa + log([Base]/[Acid])
When adjusting concentrations, one can express the base as (initial base + x) and acid as (initial acid – x) to solve for x at a target pH.
For example, if a buffer solution contains 1 M base and 2 M acid and aims for pH 7 with pKa 6, solve:
7 = 6 – log((1 + x) / (2 – x))
This algebraic adjustment yields the theoretical amounts of acid and base needed.
Limitations of the Official Buffer Capacity Definition
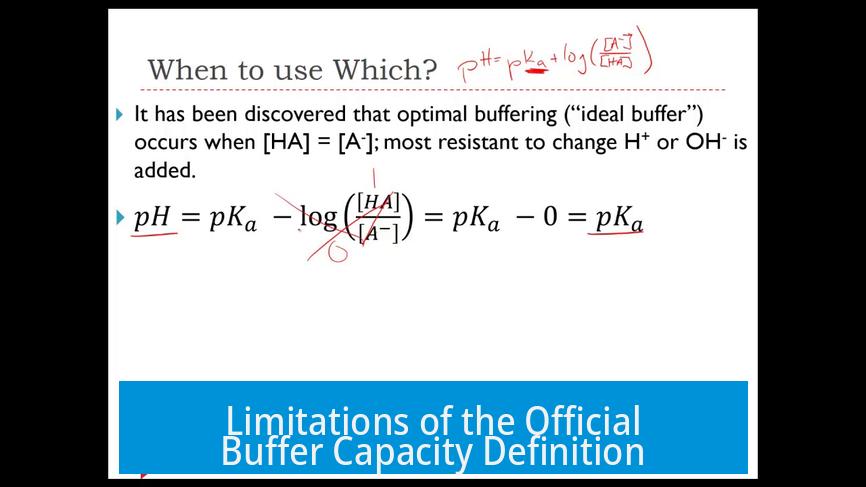
Buffer capacity measures how well a buffer resists pH change upon addition of acid or base.
Its maximum value occurs at pH = pKa, where [Acid] ≈ [Base].
However, if the pH shifts by a full unit, the remaining buffer capacity drops significantly, losing effectiveness.
In real applications, such as biochemical systems, researchers prefer to maintain pH shifts within about 0.1 units to preserve buffer integrity.
Empirical Approaches in Practice
Though algebraic calculations are possible, practitioners commonly rely on empirical methods.
- Simple mixing of acid and base
- Continuous stirring
- Real-time pH monitoring with a meter
This hands-on approach provides accurate adjustment because ionization constants vary, and slight deviations can affect pH unpredictably.
Direction-Dependence of Buffer Capacity
Buffer capacity is not symmetric. It depends on whether acid or base is being added.
Because the final buffer form often has unequal acid and base amounts, the buffer capacity differs when shifting pH toward acidic versus basic conditions.
Summary of Key Points
- The Henderson-Hasselbalch equation allows calculation of acid/base ratios for targeted pH.
- Buffer capacity peaks at pH = pKa and reduces sharply with larger pH shifts.
- Practical buffer preparation is usually empirical rather than purely theoretical.
- Buffer capacity varies based on the direction of pH change due to unequal acid-base ratios.
Theoretical Buffer Capacity Calculation: Diving Deep Without Drowning
How do you calculate theoretical buffer capacity? The short answer: Adjust the Henderson-Hasselbalch equation to find concentrations of acid and base fitting your pH goal. But hang on—it’s not quite that simple.
Calculating buffer capacity theoretically often leads chemists into a maze of equations and assumptions. The real world is messier, tricky, and offers fascinating surprises. Let’s unravel this with a pinch of fun and plenty of facts.
Why Henderson-Hasselbalch Is Your Starting Point
The classic go-to equation for buffer calculations is the Henderson-Hasselbalch equation. It elegantly relates pH, pKa, and the ratio of base to acid concentrations. You know the formula: pH = pKa + log([A−]/[HA]).
To tailor a buffer to your desired pH, imagine tweaking the acid and base amounts gently. Picture this: you start with certain concentrations of acid and base and then adjust by adding x. For example, increase your base by x (1 + x M), while reducing your acid by x (2 – x M), aiming for a target pH.
A handy example: if your base is initially 1 M and acid 2 M, and you want a pH of 7 with a pKa of 6, you arrange this:
7 = 6 – log((1 + x) / (2 – x))
Solving this gives you the exact amounts of acid and base needed. Pretty neat, right? But—and this is important—the equation assumes one form of acid and base and ignores dynamic shifts when pH changes.
The Catch: Practical Limits of Theoretical Buffer Capacity
Here’s where you might want to sit down: the official definition of buffer capacity can feel almost useless when applied directly. Why? Because if you shift your buffer’s pH by a full unit, the buffer left behind is much less capable of resisting further pH changes.
Turns out, your buffer’s power peaks at pH = pKa. The moment you veer away—say, by a whole pH unit—your buffer’s “muscle” weakens dramatically. So in reality, you’re better off working with tiny pH moves, typically around 0.1 units, especially in delicate environments like biological systems.
Why does this matter? Well, that perfect buffer you designed on paper at pH 7 might lose most of its mojo if the pH drifts even slightly.
Why Empirical Beats Theoretical (Usually)
If theoretical calculations sound intimidating, you’re in good company. Here’s a confession: in over 50 years, many chemists haven’t actually done the algebraic calculations to nail buffer concentration perfectly before mixing. Instead, they rely on empirical wisdom.
This means mixing acid and base with ionization constants within about 1.5 units apart, spinning your solution with a magnetic stirrer, and gently measuring pH with a meter. Adjustments follow based on readings, not theory alone.
So, the practical approach is a kind of “buffer crafting” in real time. Theoreticians love algebra, but even they admit it rarely replaces hands-on mixing and measuring.
Wait, Buffer Capacity Changes with Direction?
Here’s a twist you might not expect: buffer capacity is direction-dependent. If you add acid, your buffer behaves differently compared to adding base. This stems from the imbalance in acid and base concentrations in your solution.
Imagine pushing your buffer toward the acidic side. The resistance it offers won’t be the same as when you nudge it toward the base side. This directional difference complicates pure theoretical predictions.
So, the common assumption that buffer capacity is a single fixed value doesn’t hold up under closer scrutiny. Your buffer’s “charge” changes depending on how you prod it.
Wrapping It Up: Facts You Can Actually Use
- Henderson-Hasselbalch is great, but tweak it: Adjust acid/base concentrations by x to target your pH.
- Buffer capacity peaks at pH = pKa: Large pH swings (>0.1 unit) drastically shrink capacity.
- Experiment beats pure theory: Use stirring and pH measurements to refine your buffer.
- Direction matters: Buffer capacity differs when shifting pH acidic vs. basic.
Ready for a reality check? No buffer is perfect. The world of chemistry often laughs in the face of neat equations. Instead of obsessing over calculations, try this: pick a target pH close to your acid’s pKa, mix approximate amounts of acid and base, and monitor changes carefully.
This hands-on approach saves headaches and lets you feel the buffer’s “mood,” rather than guessing blindly. It’s like tuning a guitar—you don’t just calculate string tension; you listen and adjust.
Bonus Tips for Buffer Mastery
- Choose acids with pKa near your target pH. This ensures highest buffer efficiency.
- Limit pH shifts to 0.1 unit maximum. This retains high buffer capacity throughout your experiment.
- Calibrate your pH meter before starting. Accurate measurement beats perfect theory.
- Consider temperature effects. pKa can shift with temperature, altering your buffer’s effectiveness.
- Document every step. When mixing empirically, details count when repeating your success.
So next time you hear “theoretical buffer capacity,” don’t let your eyes glaze over. It’s a mix of algebra, experience, and a pinch of chemistry intuition. Just remember: the best buffer is often the one you fine-tune while stirring.
Buffer calculations are a starting point, not a finish line. Mix, measure, tweak, and you’ll get the perfect buffer suited to your scientific symphony.
What is the role of the Henderson-Hasselbalch equation in theoretical buffer capacity calculation?
The equation helps estimate pH by adjusting acid and base concentrations. It uses concentrations plus or minus a variable x to reach a target pH level for the buffer.
Why is the official buffer capacity definition limited in practical use?
Buffer capacity decreases notably after shifting pH by a full unit. The highest capacity occurs at pH equal to pKa, so large pH changes reduce its effectiveness.
How do real-world applications handle buffer capacity calculations?
Empirical methods with a pH meter and stirring are preferred over algebraic calculations. Mixing acid and base is done by trial to fine-tune pH rather than relying solely on theory.
Does buffer capacity change based on pH direction shift?
Yes, buffer capacity differs when pH shifts toward acid or base. The non-equimolar amounts of acid and base affect the capacity depending on the direction of pH change.
Can you provide an example of using Henderson-Hasselbalch for buffer preparation?
For a buffer with 1 M base and 2 M acid targeting pH 7: use the formula 7 = 6 – log((1 + x) / (2 – x)) to solve for x, adjusting concentrations accordingly.



Leave a Comment