What Is the Point of the Titer in Titration?
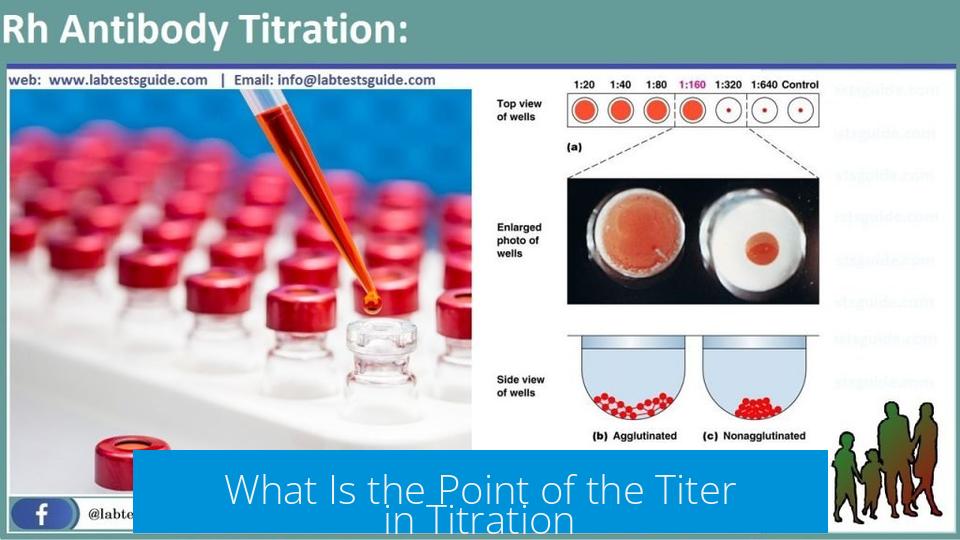
The point of the titer in titration is to provide a dimensionless factor that accurately relates the real concentration of a titrant to its nominal or labeled concentration, ensuring precise, legally valid, and comparable analytical results.
Understanding the Role of Titer
The titer corrects for slight variations in the actual concentration of a standard titrant solution. Although the standard solution concentration is labeled and assumed correct, minor discrepancies can exist. The titer accounts for these differences, aligning the effective concentration with the expected value.
Using the titer simplifies calculations by turning complex laws involving concentration into relationships involving a dimensionless parameter. This parameter acts as a proportionality factor, ensuring mathematical consistency and conserving nominal concentration and volumes.
Dimensionless Quantity for Simplification and Comparison

- The titer is a dimensionless number, simplifying various chemical calculations.
- This enables chemists to define and compare titration processes across different standards efficiently.
- It provides a consistent parameter, allowing easy adjustments without rewriting concentration values repeatedly.
Legal and Practical Importance
Many regulations require the titrant’s concentration to be verified and expressed via its titer. If the titer falls outside specified ranges, the analysis may become legally invalid. This enforces strict quality control.
Practically, measuring and applying the titer avoids laborious updates in the titrant’s labeled concentration, streamlining routine analysis workflows.
Preference and Trade-offs

Some laboratories prefer using titers due to their convenience and legal significance. Others may work directly with concentrations but may sacrifice some flexibility. Both approaches hold valid trade-offs that depend on the analytical context.
Key Takeaways
- The titer adjusts for differences between actual and nominal titrant concentration.
- It is dimensionless, simplifying chemical laws and calculations.
- The titer ensures comparability and consistency in analytical results.
- It has legal importance, affecting the validity of titration analyses.
- Using the titer streamlines lab workflows by avoiding constant concentration updates.
Titration: What Is the Point of the Titer?
Simply put, the titer is a dimensionless number that tells you the true strength of your titrant solution relative to a standard, and it plays a crucial role in ensuring accurate and legally valid titration results. But that’s just the tip of the iceberg. Let’s dive deeper into why the titer matters, how it simplifies complex equations, and why chemists, regulators, and even the laziest lab techs love it.
What Exactly Is a Titer? And Why Haven’t You Heard of It?
“Unless I am way out of the loop, ‘titer’ isn’t commonly used,” some might say. You’re not alone if this term sounds more like something from a vintage chemistry textbook rather than your everyday lab chat. But the titer is pretty important, especially in quality control labs and legal standards setting.
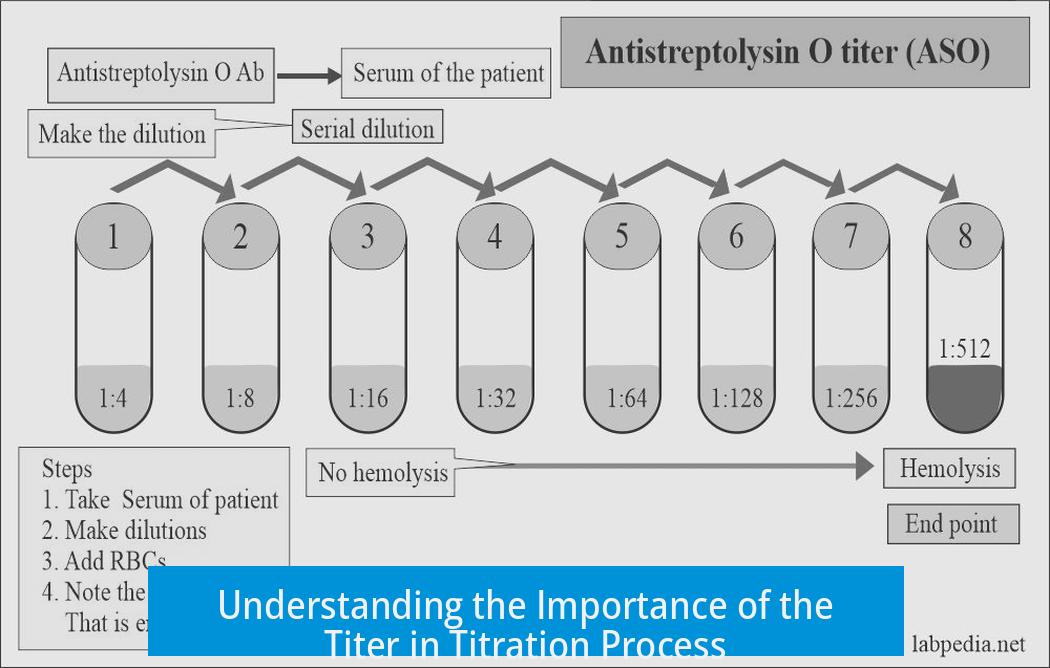
In titration, the term “titer” refers to a factor that adjusts the nominal concentration of a standard solution to its actual, true concentration. In other words, if you think your titrant solution is exactly 0.1 M, the titer tells you if that is true or if the actual concentration is slightly more or less. It’s a way to correct discrepancies between the expected and actual strengths.
The Role of Standard Solutions and Concentration in Titration
You might ask, “Why can’t we just use the concentration on the bottle label?” Here’s the catch. The standard solution is prepared with a nominal concentration—what’s “on the label.” But lab conditions, reagent purity, and preparation errors mean the real concentration might differ.
This is where the titer shines. Since your analyte’s exact concentration is unknown, the titrant’s exact strength must be known precisely. That’s why labs use standard solutions measured accurately and periodically verified—often by titration against a primary standard. The titer provides a correction factor so you don’t blindly trust that the concentration on the bottle is gospel.
Why Use the Titer? The Hidden Benefits
Dimensionless Quantity Simplifies Complex Chemistry
The titer is a “dimensionless number.” That sounds fancy, but it simply means it’s a pure number without units. This removes complications in equations where mixing units leads to messy math. Without units, the titer lets chemists simplify formulas and calculations, making it “one less headache” in complex titration laws.
Enables Comparison with a Common Scale
Think of the titer as a universal translator for titration results. Say you want to compare two processes or solutions from different labs or batches. If everyone uses nominal concentrations, those numbers may not tell the full story.
By applying the titer—a proportionality factor—you rescale measurements onto a level playing field. This approach “conserves” the law mathematically by treating nominal concentration and volume as constants but allows for a variable that accurately matches reality. It’s like swapping confusing local currencies for one stable, universal money.
The Legal and Practical Weight of the Titer
Here’s where things get serious. The titer isn’t just a lab curiosity; it’s baked into many laws and regulations. If the titer is outside specified limits—say, below or above certain values—your entire titration analysis could be deemed legally invalid. That’s not just a theoretical risk. It affects industries from pharmaceuticals to environmental testing.
On the practical side, measuring the titer keeps chemists from rewriting concentration values again and again. With one “constant law” that allows a variable titer factor, analysts can apply a cleaner, simpler method. Sound lazy? Maybe. But in a lab setting where time and accuracy are key, it’s smart.
Preferences, Trade-offs, and Real-World Choices
Of course, there’s no one-size-fits-all approach. Some chemists prefer to use fixed concentrations with no additional correction factor and accept minor inaccuracies. Others insist on including the titer to achieve top-shelf precision and legal certainty. Both ways have their merits.
The choice often boils down to context: Is this a casual lab test or a regulatory-required analysis? Do you prioritize speed or absolute accuracy? A careful balance is necessary, and the titer offers a powerful tool to hit that balance.
Lessons from Experience: How I Learned to Love the Titer
When I first started my lab work, titration felt like a chore with endless calculations and guesswork. I used standard concentrations blindly—more out of habit than understanding. Then one day, a batch failed a regulatory inspection because the titrant concentration was off slightly.
After embracing the concept of the titer, retesting, and recalculating with this correction factor, my results became rock-solid. It felt like upgrading from a rusty calculator to a sleek smartphone. Suddenly, data matched expectations, compliance was smooth, and my days in the titration trenches got easier.
Practical Tips for Working with Titer in Titration
- Always verify your standard solution’s titer regularly. It changes with storage conditions and age.
- Use the titer factor as a multiplier to adjust nominal concentrations rather than rewriting formulas.
- Focus on legal limits. If your titration results hinge on regulatory compliance, knowing the titer isn’t optional.
- Record the titer and when it was measured. Transparency helps audits and future reference.
- Communicate findings clearly. When sharing data, specify if the titer was applied.
Final Thoughts
The titer might seem like an obscure shadow actor behind the scenes, but it’s central to titration accuracy and legal validity. It corrects your standard solution’s nominal concentration, simplifies complex chemical laws, and offers a dimensionless number for fair comparisons across labs and batches.
If you want titration results that stand up to scrutiny—whether legal, scientific, or practical—the titer is your unsung hero. Next time you hear “titer,” you won’t just nod politely. You’ll know it’s the key to precision, simplicity, and trustworthiness in chemistry.
What is the main purpose of determining the titer in titration?
The titer helps to adjust the nominal concentration of the titrant. It corrects for any deviation from the labeled concentration. This ensures accuracy in quantitative analysis.
Why is the titer considered a dimensionless quantity?
The titer is a pure number without units. It simplifies complex calculations and laws by acting as a proportionality factor. This makes comparisons between different standards easier.
How does titer affect legal compliance in chemical analysis?
Many regulations specify acceptable titer ranges for titrants. If the titer falls outside these ranges, the analysis results may not be legally valid. Measuring titer ensures regulatory compliance.
Why might one prefer using titer over recalculating concentration each time?
Using a constant law with a varying titer is often simpler. It avoids frequently rewriting concentration values. This convenience can save time and reduce errors during analysis.
Can the titer vary between different titrant batches?
Yes. Even standard solutions can have slight concentration differences. Measuring titer accounts for these variations to maintain precision in titration results.




Leave a Comment