What Are Resonance Structures?
Resonance structures are multiple Lewis dot representations of a molecule that differ only in the placement of electrons, providing a better description of its bonding than any single Lewis structure can. These structures serve as corrections to the simple depiction of electrons in Lewis diagrams.
Understanding Lewis Structures and Resonance
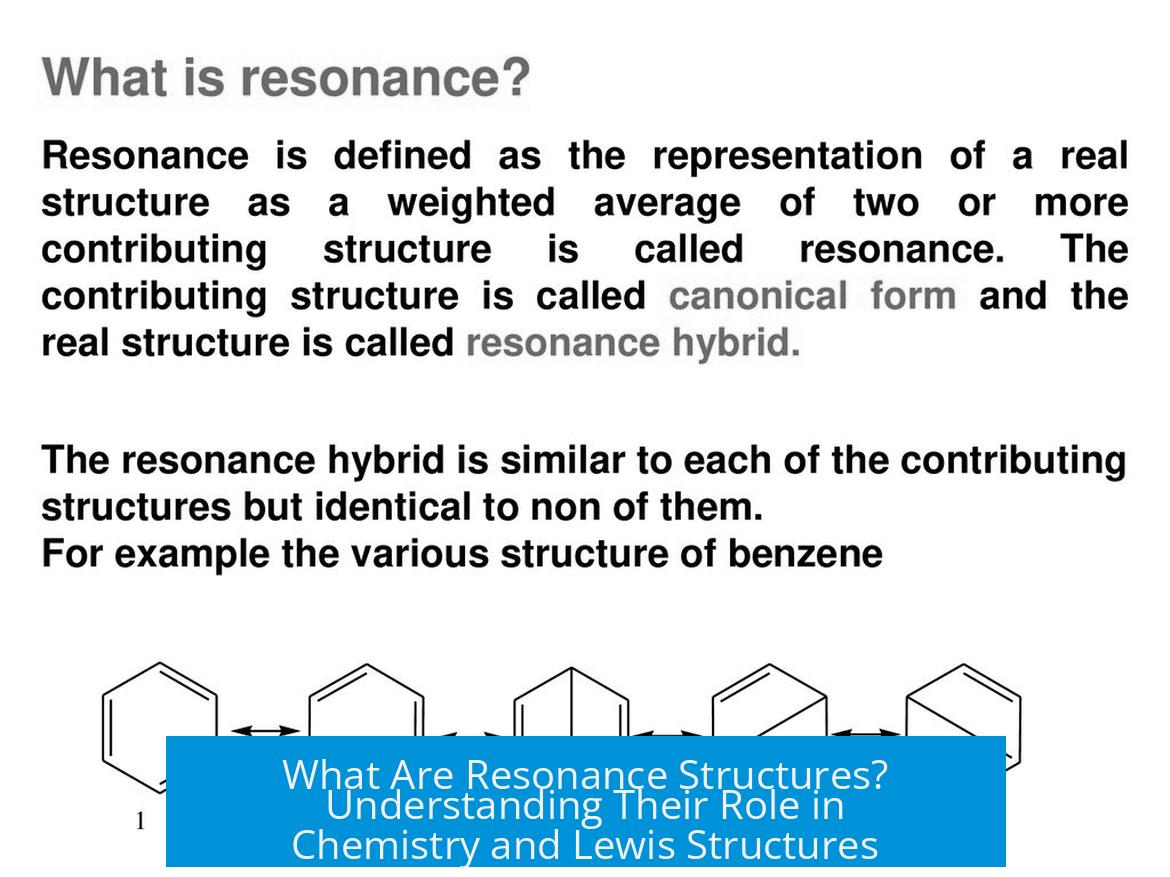
Lewis structures often fail to capture the true electronic distribution in a molecule. Some molecules have two or more equivalent Lewis structures—examples include benzene and the carboxylate ion. These equivalent structures suggest that the actual electron arrangement is not represented by one structure alone.
The Resonance Hybrid Concept
Resonance structures are combined into a resonance hybrid. This hybrid acts as an average of all contributing Lewis structures. Importantly, this average represents a static electron distribution. It is not a rapid switching or oscillation between the forms. Because of this, the term “resonance” can be misleading.
Non-Equivalent Resonance Structures
Sometimes resonance structures are not equally stable but close in energy. In these cases, the resonance hybrid is biased toward the lower energy structure. This weighted average still offers a more accurate representation of bonding than any single structure.
Practical Example: Amide Bonds
The planarity of amide bonds in proteins arises from resonance. Here, the resonance hybrid stabilizes the bond and explains the observed structural rigidity. The partial double bond character created by resonance restricts rotation, enforcing planarity.
Summary of Key Points
- Resonance structures improve on simple Lewis dot electron depictions.
- They involve equivalent or close-in-energy Lewis structures.
- The resonance hybrid is a static average, not oscillation.
- Biased hybrids occur if structures differ in energy.
- Resonance explains molecular properties such as planarity in amides.



Leave a Comment