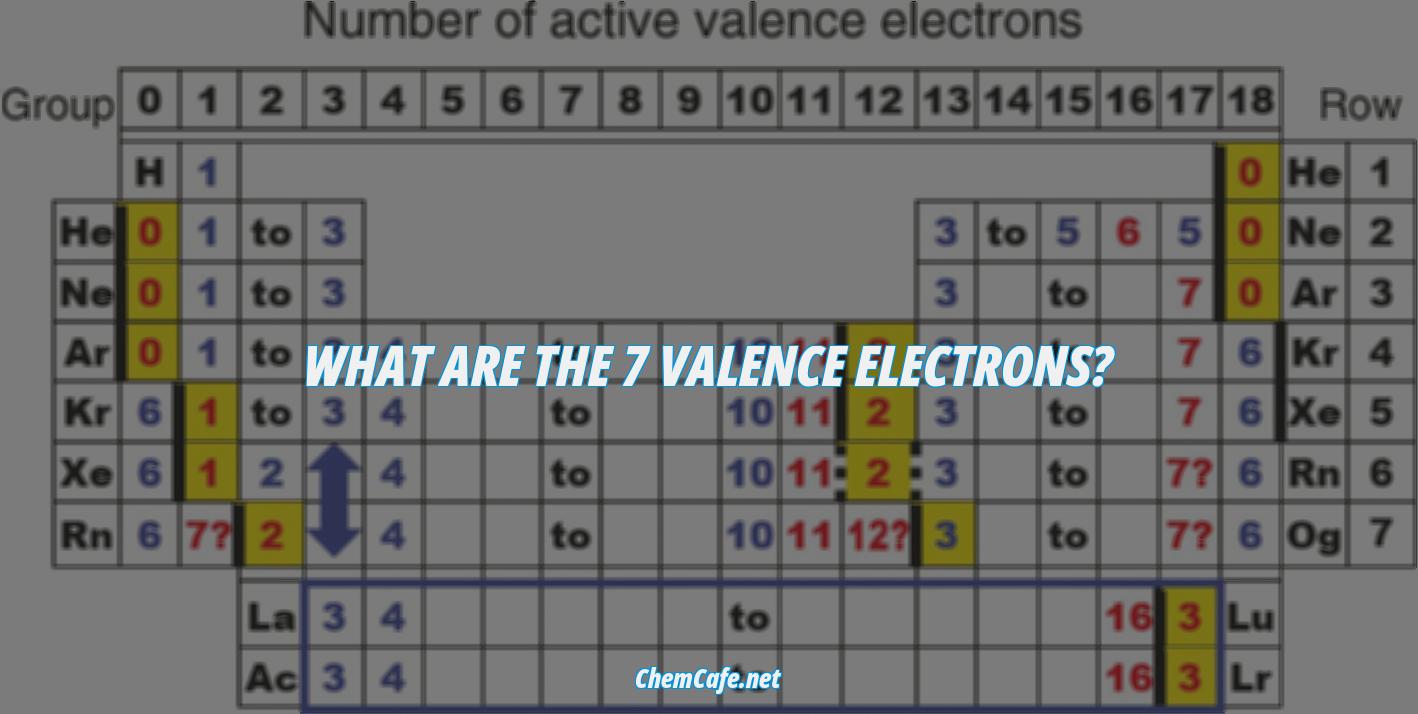
Valence electrons play an important role in the chemical behavior of an atom. They are the number of electrons present in the outermost shell of an atom, and are essential in determining the reactivity of the atom and its ability to form compounds. In this article, we’ll discuss the seven valence electrons, explore the elements that have them, and explain how they interact with the periodic table.
Atoms of the Group 7 elements, including fluorine, chlorine, bromine, iodine, and astatine, all have seven valence electrons in their outer shell. This means that these elements all have similar chemical reactions, and are all classified as halogens. In addition, these elements all form ionic compounds with phosphorous.
Carbon atoms, however, have four valence electrons each. This makes carbon a unique element, as it is the only element in Group 4 of the periodic table with this number of valence electrons. This is why carbon is often referred to as the “building block of life”, as it is the basis of organic chemistry and all living things.
Valence electrons are also important in determining the stability of an atom. An atom with seven valence electrons is considered stable, as it has not formed a ion yet. Conversely, an atom with more than seven valence electrons is considered unstable, and may form an ion if it gains or loses electrons.
In conclusion, the seven valence electrons are essential in determining the chemical behavior of an atom. They are found in the outer shell of atoms of Group 7 elements, and form ionic compounds with phosphorous. Carbon atoms, on the other hand, have four valence electrons each, making them a unique element. Finally, the stability of an atom is determined by its valence electrons, with seven being the most stable.
What are the 7 valence electrons?
Valence electrons are the outermost electrons of an atom, and they are responsible for the chemical reactions that occur in a molecule. They are the electrons that determine whether a molecule will form a covalent or an ionic bond. Knowing the number of valence electrons an atom has can help you predict its reactivity and the types of compounds it will form.
The 7 valence electrons are the electrons in the outermost shell of an atom, and they are responsible for the atom’s chemical properties. These electrons are what make the atom reactive and give it its unique properties. All atoms in the same group of the periodic table have the same number of valence electrons. Group 7 elements, which are also known as the halogens, all have 7 valence electrons. This includes the elements fluorine, chlorine, bromine, iodine, and astatine.
Do Group 7 elements have 7 electrons?
Yes, atoms of group 7 elements all have seven electrons in their outer shell. This means that the halogens all have similar chemical reactions. They are all very reactive, and they form ions when they react with other elements. For example, when chlorine reacts with sodium, it forms a chloride ion, which is an anion with a charge of -1.
Does group 17 have 7 valence electrons?
No, group 17 elements do not have 7 valence electrons. Group 17 elements are also known as the halogens, but they only have 6 valence electrons. These include the elements fluorine, chlorine, bromine, iodine, and astatine.
What elements has 7 electrons?
The Group 7 elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven electrons in the outer shell. These elements are all very reactive and form ions when they react with other elements.
Which of these has a valence electron of 7?
F, Cl and Br are the elements each having seven valence electrons. These elements are all very reactive and form ions when they react with other elements. They are all part of the halogen family and have similar chemical properties.
Valence Electrons and the Periodic Table
Valence electrons are the outermost electrons of an atom, and they are responsible for the chemical reactions that occur in a molecule. Knowing the number of valence electrons an atom has can help you predict its reactivity and the types of compounds it will form. In the periodic table, elements in the same group have the same number of valence electrons. Group 7 elements all have 7 valence electrons.
Which family has 7 electrons in valence shell?
The halogens have seven electrons in the valence shell and form ionic compounds with phosphorous. When a halogen reacts with a metal, the halogen gains an electron from the metal and forms an ionic bond. This is because the halogens have a higher electronegativity than the metals, so they are able to pull electrons away from other atoms.
Does carbon have 7 valence electrons?
No, carbon atoms have 4 valence electrons each. Carbon can form covalent bonds with other atoms, which means that it shares electrons with other atoms. Carbon is a very versatile element and can form many different types of compounds.
Is an atom with 7 valence electrons stable?
Yes, an atom with 7 valence electrons is stable. Valence electrons are the number of electrons present in the outermost shell of an atom. Now, the last shell of chlorine atom has 7 electrons in it. Therefore, there are 7 valence electrons present in an chlorine atom. Here, stable means that atom has not formed a ion yet.
In conclusion, the 7 valence electrons are the electrons in the outermost shell of an atom. All atoms in the same group of the periodic table have the same number of valence electrons. Group 7 elements, which are also known as the halogens, all have 7 valence electrons. These include the elements fluorine, chlorine, bromine, iodine, and astatine. Knowing the number of valence electrons an atom has can help you predict its reactivity and the types of compounds it will form. An atom with 7 valence electrons is stable as long as it has not formed an ion.
Which elements has 7 valence electrons?
Valence electrons are the electrons located in the outermost shell of an atom. They are responsible for the chemical properties of atoms and the elements they form. In general, each element has a different number of valence electrons, which is determined by its position in the periodic table. The number of valence electrons determines how an atom interacts with other atoms and how it forms chemical bonds.
Do Group 7 elements have 7 electrons?
Group 7 elements, which are also known as the halogens, all have seven electrons in their outer shell. This means that all of these elements have the same number of valence electrons, which is seven. This is why the halogens have similar chemical reactions.
Does group 17 have 7 valence electrons?
Group 17 elements are also known as the halogens. These elements also have seven electrons in their outer shell, which means that they have seven valence electrons as well. This is why the halogens have similar chemical reactions.
What elements has 7 electrons?
The Group 7 elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven electrons in the outer shell. This means that these elements all have seven valence electrons.
Which of these has a valence electron of 7?
F, Cl and Br are the elements each having seven valence electrons. These three elements are the most reactive of the halogens, since they have the highest number of valence electrons.
Valence Electrons and the Periodic Table
The valence electrons are the electrons in the outermost electron shell of an atom. That is why elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration #s^2p^6#. This tendency is called the octet rule, because the bonded atoms have eight valence electrons.
Which family has 7 electrons in valence shell?
The halogens have seven electrons in the valence shell and form ionic compounds with phosphorous. This is due to the high number of valence electrons, which allows the halogens to easily form ionic bonds with other elements.
In conclusion, the Group 7 elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven valence electrons. These elements are all part of the halogen family and react in similar ways due to their high number of valence electrons. This is why the halogens are grouped together in the Periodic Table and form ionic compounds with phosphorous.
Which family has 7 valence electrons?
Atoms are composed of particles called protons, neutrons, and electrons. Electrons are located in energy levels that orbit the nucleus of an atom, and the valence electrons are located in the outermost energy level. Valence electrons are important in chemical reactions and form the basis of the periodic table of elements. Knowing how many valence electrons an element has is essential for understanding how it behaves in different chemical reactions.
Group 17 Elements Have 7 Valence Electrons
The periodic table is arranged by atomic number, and each element has a unique atomic number. Group 17 elements, also known as the halogens, have seven valence electrons. These include fluorine, chlorine, bromine, iodine, astatine, and tennessine. These elements are non-metals, and their outermost shells are already full, so they are very stable.
Group 18 Elements Have 8 Valence Electrons
Group 18 elements, also known as the noble gases, have eight valence electrons. These include helium, neon, argon, krypton, xenon, and radon. Since these elements already have a full outer energy level, they are very stable and unreactive. This makes them useful in a variety of applications, such as filling light bulbs and preserving food.
Group 7 Elements Have 7 Valence Electrons
Group 7 elements, also known as the alkali metals, have seven valence electrons. These include lithium, sodium, potassium, rubidium, cesium, and francium. These elements are all metals, and they are very reactive. They tend to take one electron and form a negative ion, giving them a valency of -1.
Group 4 Elements Have 4 Valence Electrons
Group 4 elements, also known as the carbon group, have four valence electrons. These include carbon, silicon, germanium, tin, and lead. These elements are all non-metals, and they tend to form covalent bonds.
Group 11 Elements Have 1 Valence Electron
Group 11 elements, also known as the coinage metals, have one valence electron. These include copper, silver, and gold. These elements are all metals, and they are very reactive. They tend to take one electron and form a positive ion, giving them a valency of +1.
Conclusion
Atoms are composed of protons, neutrons, and electrons, and the valence electrons are located in the outermost energy level. Knowing how many valence electrons an element has is essential for understanding how it behaves in different chemical reactions. Group 17 elements have seven valence electrons, Group 18 elements have eight valence electrons, Group 7 elements have seven valence electrons, Group 4 elements have four valence electrons, and Group 11 elements have one valence electron.
What elements have 7 valence?
Valence electrons are the electrons present in the outermost shell of an atom and determine the chemical and physical properties of the elements. Each element has a certain number of valence electrons, and this number can be found on the periodic table. In Group 7A, also known as the halogens, all the elements have seven valence electrons in their highest-energy orbitals (ns2np5).
The halogen group includes elements such as fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At). These elements all have seven electrons in their outer shell, which makes them reactive and highly oxidizing. Their reactivity is due to the fact that they are always trying to complete their electron shells by forming compounds.
What element has 7 valence electrons?
Fluorine (symbol F) is found in column 7 on the periodic table and is the only element in Group 7A with seven valence electrons. It is a non-metal and is the most reactive and electronegative element on the periodic table. Fluorine has the atomic number 9 and is a pale yellow gas that is highly poisonous.
Chlorine (Cl) is also found in Group 7A and has seven valence electrons. It is a non-metal and is a greenish-yellow gas that is also highly poisonous. Chlorine is extremely reactive and can form compounds with most elements.
Bromine (Br) is the third element in Group 7A with seven valence electrons. It is a non-metal and is a reddish-brown liquid that is highly toxic. Bromine is also highly reactive and can form compounds with most elements.
What is the name “halogen”?
The name “halogen” means “salt former”, derived from the Greek words halo- (“salt”) and -gen (“formation”). Halogens are highly reactive and can form compounds with most elements, which is why they are often referred to as salt formers.
What is the group of elements that have 7 valence electrons?
The Group 7A elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven electrons in the outer shell. These elements are highly reactive and can form compounds with most elements.
Which of these has a valence electron of 7?
Fluorine (F), chlorine (Cl) and bromine (Br) are the elements each having seven valence electrons. All of these elements are non-metals and can form compounds with most elements.
Valence Electrons and the Periodic Table
Valence electrons are the number of electrons present in the outermost shell of an atom. The number of valence electrons in each element can be determined by looking at the periodic table. The elements in Group 7A all have seven electrons in their outer shell, which makes them highly reactive and able to form compounds with most elements.
Which family has 7 electrons in valence shell?
Halogens have seven electrons in the valence shell and form ionic compounds with phosphorous. Halogens are highly reactive and can form compounds with most elements, which is why they are often referred to as salt formers.
In conclusion, the Group 7A elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven electrons in the outer shell. These elements are highly reactive and can form compounds with most elements. Fluorine (F), chlorine (Cl) and bromine (Br) are the elements each having seven valence electrons. Halogens have seven electrons in the valence shell and form ionic compounds with phosphorous. Valence electrons are the number of electrons present in the outermost shell of an atom and determine the chemical and physical properties of the elements.
Do halogens have 7 electrons?
Halogens are a group of elements located in Group 17 of the periodic table. All of the elements in this group have seven electrons in their outermost shell. This characteristic is what makes them so reactive and gives them their name – halogen, which means “salt-forming.”
What Are Valence Electrons?
Valence electrons are the outermost electrons of an atom and are responsible for an atom’s reactivity. Atoms try to fill their outermost shells with electrons and this is why they react with other elements. Halogens have seven valence electrons, which means they are very “eager” to gain one electron to complete their outermost shell.
How Do Halogens React?
Halogens are highly reactive nonmetallic elements in group 17 of the periodic table. This reactivity is due to their seven valence electrons. When a halogen gains an electron, it forms an anion with a -1 charge known as a halide. These halides are fluoride (F-), chloride (Cl-), bromide (Br-) and iodide (I-).
In their elemental form, the halogens form diatomic molecules, X2, connected by single bonds. When combined with other nonmetals, the halogens form compounds through covalent bonding.
Why Do Halogens Have 7 Valence Electrons?
Halogens are one electron away from having a full octet of eight electrons. Since these elements cannot gain any more electrons, they become highly reactive in an effort to fill their outermost shells. The reactivity of the halogens is what makes them so useful in a variety of applications, from medical treatments to industrial processes.
Halogens are a group of elements located in Group 17 of the periodic table. All of the elements in this group have seven electrons in their outermost shell. This is what makes them so reactive and gives them their name – halogen, which means “salt-forming.” Halogens are highly reactive nonmetallic elements in group 17 of the periodic table and have seven valence electrons. This reactivity is due to their seven valence electrons and is what makes them so useful in a variety of applications, from medical treatments to industrial processes.
What group has 7 valence electrons?
Valence electrons are the electrons that are present in the outermost shell of an atom. These electrons largely determine the chemical properties of an element and can be used to predict how an element will interact with other elements. The Group 7 elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven electrons in the outer shell, and are therefore known as the halogens.
What are Valence Electrons?
Valence electrons are the electrons that are present in the outermost shell of an atom. These electrons largely determine the chemical properties of an element and can be used to predict how an element will interact with other elements.
In a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element’s chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell.
Do Group 7 Elements Have 7 Valence Electrons?
Yes, atoms of group 7 elements all have seven electrons in their outer shell. This means that the halogens all have similar chemical reactions.
Does Group 17 Have 7 Valence Electrons?
No, group 17 elements (the halogens) have seven valence electrons, but group 17 elements (the noble gases) have a full outer shell, so they do not have any valence electrons.
What Elements Have 7 Electrons?
The Group 7 elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven electrons in the outer shell.
Which of These Has a Valence Electron of 7?
F, Cl and Br are the elements each having seven valence electrons.
Valence Electrons and the Periodic Table
The number of valence electrons an element has can be determined by looking at the periodic table. The elements in the same group typically have the same number of valence electrons. For example, the Group 7 elements (the halogens) all have seven valence electrons.
Which Family Has 7 Electrons in Valence Shell?
Halogens have seven electrons in the valence shell and form ionic compounds with phosphorous. The halogens are the most reactive of the nonmetals and they are often found in nature in their molecular form.
In conclusion, the Group 7 elements – fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) – have seven electrons in the outer shell. This means that the halogens have similar chemical reactions and can form ionic compounds with phosphorous. Valence electrons are an important part of understanding how elements interact with each other and can be used to predict how an element will bond with other elements.



Leave a Comment