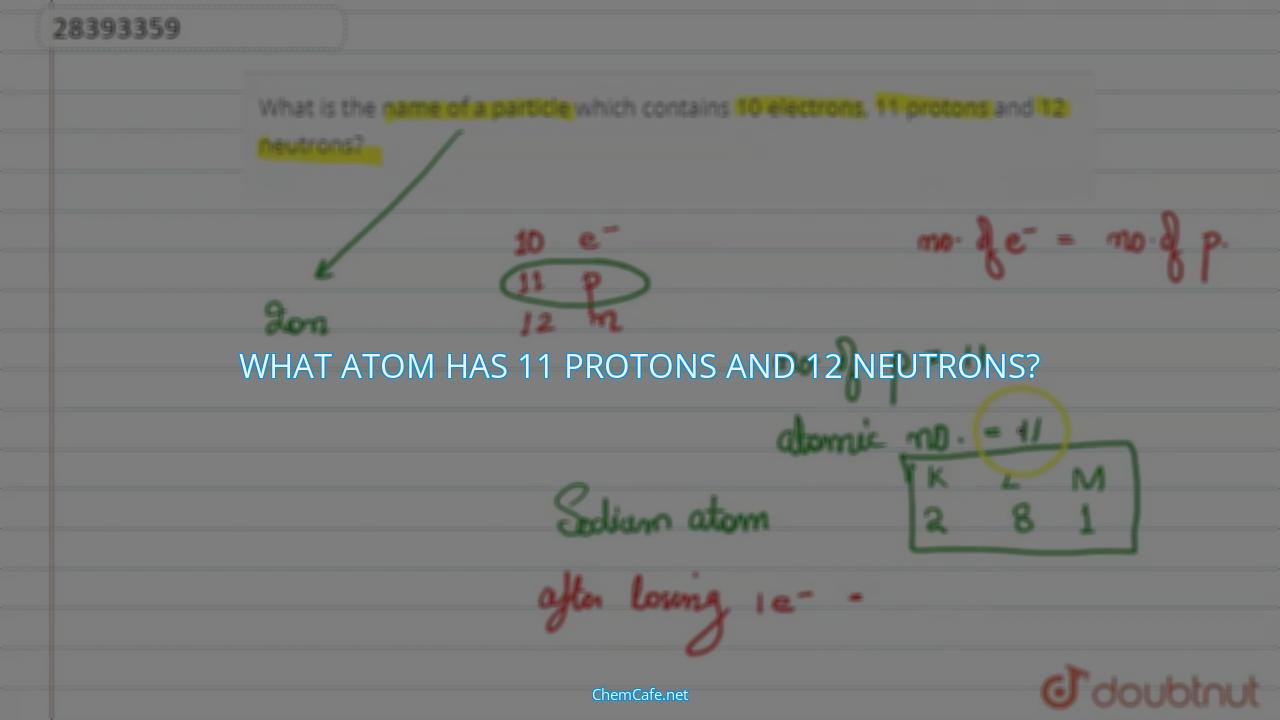
Do you ever wonder what the makeup of the atom is? What is the structure of the atom’s nucleus? Well, atoms are made up of protons and neutrons, and the number of each can vary depending on the element. One particular element that has 11 protons and 12 neutrons is sodium.
Sodium is a chemical element found naturally in the Earth’s crust and is the sixth most abundant element. Sodium is an alkali metal and is found in a variety of minerals, including its most common form, table salt. Sodium is an important element for many everyday uses, including for cooking, food preservation, and the production of glass, paper, and soap.
Sodium can exist in a variety of forms, including isotopes. An isotope is an atom of the same element with a different number of neutrons in its nucleus. There are three isotopes of carbon, all with 6 protons, but with different numbers of neutrons. The atom on the left has 6 neutrons, the atom in the middle has 7 neutrons, and the atom on the right has 8 neutrons.
Isotopes of sodium can be identified by their mass, which is the total number of protons and neutrons. The isotope of sodium that has 11 protons and 12 neutrons is sodium-23. Sodium-23 is an important isotope in the medical industry and is used in tracer studies, to diagnose and treat cancer, and in the diagnosis of kidney and heart problems.
So, if you ever wondered what atom has 11 protons and 12 neutrons, it is sodium-23. This isotope is an important element in many everyday uses and in the medical industry. It is important to understand the structure of atoms, what isotopes are, and how they are formed in order to fully appreciate the importance of this element.
What atom has 11 protons and 12 neutrons?
Atoms are the basic building blocks of the universe. Every atom is made up of protons, neutrons and electrons. The number of protons and neutrons determines the type of atom, and the number of electrons determines its charge.
One particular atom that has 11 protons and 12 neutrons is the isotope of sodium, known as Na-23. This isotope of sodium is not the most common form of sodium, but it does exist in nature. It is a stable isotope, meaning that it does not undergo radioactive decay.
What is an Isotope?
An isotope is a form of an element that has the same number of protons but a different number of neutrons. This difference gives the isotope a different mass than other atoms of the same element. All isotopes of an element have the same chemical properties, but their physical properties may differ.
For example, the isotope of carbon known as carbon-12 (C-12) has 6 protons and 6 neutrons, while the isotope of carbon known as carbon-14 (C-14) has 6 protons and 8 neutrons. Both atoms have the same chemical properties, but carbon-14 has a different mass than carbon-12.
What is Na-23?
Na-23 is an isotope of sodium that has 11 protons and 12 neutrons. This makes it slightly heavier than the most common isotope of sodium, Na-22, which has 11 protons and 11 neutrons. Because of its higher mass, Na-23 is more stable than Na-22 and does not undergo radioactive decay.
What other factors are related to Na-23?
Aside from its 11 protons and 12 neutrons, Na-23 is also made up of 11 electrons. This is because atoms are electrically neutral, so they must have the same number of positively charged protons as negatively charged electrons.
Na-23 also has a different mass than other isotopes of sodium. Its mass is 23, which is the total number of protons and neutrons. In addition, Na-23 has a different atomic radius than other isotopes of sodium. This is because the number of protons and neutrons affects the size of the atom.
Na-23 is an isotope of sodium that has 11 protons and 12 neutrons. This makes it slightly heavier than the most common isotope of sodium, Na-22. Na-23 is also made up of 11 electrons, and has a different mass and atomic radius than other isotopes of sodium.
What element has 12 neutrons and 11 protons?
The answer is sodium, with the isotope of Na-23. Sodium is an alkali metal that is found in the Earth’s crust and is used in many industries, such as baking and food processing. It is also found in many living organisms, including humans.
What is an isotope?
An isotope is an atom of the same element that has a different number of neutrons in its nucleus. Isotopes of an element have the same number of protons, but different numbers of neutrons. This means that they have different atomic masses, but still have the same chemical properties.
What is the isotope of sodium with 11 protons and 12 neutrons?
The isotope of sodium with 11 protons and 12 neutrons is Na-23, which has an atomic mass of 23. This isotope is the most abundant isotope of sodium, making up almost 92% of all naturally occurring sodium. It is a stable isotope, meaning it does not undergo radioactive decay.
What are the properties of Na-23?
Na-23 has a melting point of 97.8 °C and a boiling point of 883.0 °C. It is a soft, silvery-white metal and is highly reactive. It reacts with water to form sodium hydroxide, a strong base, and hydrogen gas. It also reacts with chlorine gas to form sodium chloride, or table salt.
What is the significance of Na-23?
Na-23 is an important element in many industries. It is used to produce baking soda, which is used in baking, as well as in food processing and cleaning products. Sodium is also used to produce sodium hydroxide, which is used in the manufacture of paper, soap, and detergents. Sodium is also essential for life, as it is found in many living organisms.
Na-23 is an isotope of sodium that has 11 protons and 12 neutrons. It has an atomic mass of 23 and is the most abundant isotope of sodium. It is a stable isotope and has a melting point of 97.8 °C and a boiling point of 883.0 °C. Na-23 is used in many industries, and is also essential for life.
Which element has 12 neutrons and 11 electrons?
Atoms are the smallest particles of an element and are made up of three subatomic particles: protons, neutrons, and electrons. The number of protons in an atom determines its atomic number and its identity as a particular element. The number of electrons in an atom is equal to the number of protons, but the number of neutrons can vary. In this article, we will answer the question, which element has 12 neutrons and 11 electrons?
Atomic Structure
Atoms are composed of three subatomic particles, protons, neutrons, and electrons. The protons and neutrons make up the nucleus, or the center of the atom, while the electrons make up the electron cloud around the nucleus. Protons have a positive charge, electrons have a negative charge, and neutrons have no charge. The number of protons in an atom determines its atomic number, which is the number that is used to identify a particular element.
Isotopes
Atoms of the same element can have different numbers of neutrons in their nuclei. These atoms are called isotopes. For example, carbon has three isotopes, Carbon-12, Carbon-13, and Carbon-14. All three isotopes have 6 protons, but Carbon-12 has 6 neutrons, Carbon-13 has 7 neutrons, and Carbon-14 has 8 neutrons.
Ions
Atoms are electrically neutral, so they have the same number of negatively charged electrons as positively charged protons. However, some atoms can gain or lose electrons, resulting in an atom with a net charge. These atoms are called ions. For example, sodium is one of several atoms that easily donates an electron, leaving it with 11 protons, 11 electrons, and 12 neutrons. This makes it a sodium ion with a plus one charge.
Answer
The answer to the question, which element has 12 neutrons and 11 electrons, is sodium. Sodium is one of the few atoms that can easily donate an electron, leaving it with 11 protons, 11 electrons, and 12 neutrons. This makes it a sodium ion with a plus one charge. Other elements, such as potassium and magnesium, also have 11 electrons and 12 neutrons, but they typically gain an electron, resulting in a negative charge.
Atoms are the smallest particles of an element and are made up of three subatomic particles: protons, neutrons, and electrons. The number of protons in an atom determines its atomic number and its identity as a particular element. The number of electrons in an atom is equal to the number of protons, but the number of neutrons can vary. An atom with 11 protons, 11 electrons, and 12 neutrons is an atom of sodium. This makes it a sodium ion with a plus one charge. Other elements, such as potassium and magnesium, also have 11 electrons and 12 neutrons, but they typically gain an electron, resulting in a negative charge.
What has 12 neutrons and 11 electrons?
Atoms consist of a nucleus, which is made up of protons and neutrons, and electrons that move around the nucleus. The number of protons in an atom is referred to as its atomic number and the number of neutrons is referred to as its mass number. A neutral atom has the same number of protons and electrons.
Sodium (Na) has an atomic number of 11 and a mass number of 23
The atomic number of sodium is 11, meaning that it has 11 protons in its nucleus. The mass number of 23 tells us that it has 12 neutrons in its nucleus. The number of electrons outside the nucleus is equal to the number of protons, so in this case, 11 electrons. Therefore, sodium has 11 protons, 12 neutrons, and 11 electrons.
What is an isotope?
An isotope is an atom of an element with the same number of protons but different numbers of neutrons. Isotopes of an element have the same chemical properties but different physical properties. For example, hydrogen has three naturally occurring isotopes; protium (1 proton, 0 neutrons), deuterium (1 proton, 1 neutron), and tritium (1 proton, 2 neutrons).
An isotope of sodium contains 11 protons and 12 neutrons
An isotope of sodium contains 11 protons and 12 neutrons. The atom also contains 11 electrons, as the number of electrons is equal to the number of protons. This isotope of sodium is known as sodium-23, as it has a mass number of 23. It is by far the most abundant isotope of sodium, making up 99.6% of all naturally occurring sodium.
The importance of isotopes
Isotopes are important for many reasons. They are used in a variety of applications, such as medical imaging, radiotherapy, and tracer studies. Isotopes are also used in scientific research, for example to study the behavior of atoms and molecules. Isotopes are also used to date ancient artifacts and to study the history of the Earth.
In conclusion, an atom of sodium has 11 protons, 12 neutrons, and 11 electrons. An isotope of sodium contains 11 protons and 12 neutrons, and it also contains 11 electrons. Isotopes are important for many applications, such as medical imaging, radiotherapy, and tracer studies. Finally, isotopes are also used to date ancient artifacts and to study the history of the Earth.
What atom has 11 protons 12 neutrons and 11 electrons?
Atoms are the smallest particles of an element that can exist in an isolated form. Each atom is made up of one or more protons and neutrons in the nucleus, surrounded by a cloud of electrons. In order for the atom to be neutral, the number of protons in the nucleus must equal the number of electrons outside the nucleus. Different elements have different numbers of protons, and this is what gives them their unique chemical properties.
Sodium (Na) has an atomic number of 11 and a mass number of 23
Sodium has an atomic number of 11, which means it has 11 protons in its nucleus. The mass number of sodium is 23, which is the sum of the number of protons and neutrons in the nucleus. So, if sodium has 11 protons, then it must have 12 neutrons in its nucleus. Number of protons = number of electrons, so sodium also has 11 electrons outside the nucleus.
What is an isotope?
An isotope is an atom of an element with the same number of protons but different numbers of neutrons. These isotopes can have different masses and different chemical properties, even though they are still the same element. The electrons help to balance the positive charge of the protons with their own negative charge, sometimes creating ions if the number of electrons does not equal the number of protons.
Answer and Explanation
An atom with 11 protons, 11 electrons, and 12 neutrons is an atom of sodium. The element is determined by the number of protons in the nucleus of the atom, so sodium has 11 protons and is therefore a sodium atom. Since the number of protons is the same as the number of electrons, the atom also has 11 electrons outside the nucleus. The mass number of sodium is 23, which is the sum of the number of protons and neutrons in the nucleus. So, the number of neutrons in the nucleus of the sodium atom is 12.
In summary, an atom with 11 protons, 12 neutrons, and 11 electrons is an atom of sodium. The number of protons determines the element, and the number of electrons and neutrons determines the mass and charge of the atom. Isotopes are atoms of the same element but with different numbers of neutrons, and these can have different chemical properties.
What atom has 11 protons and 11 electrons?
Atoms are the building blocks of matter, and they are composed of protons, neutrons, and electrons. The protons carry a positive charge, the neutrons carry no charge, and the electrons carry a negative charge. The number of protons an atom has determines what element it is, and the number of electrons helps to balance the positive charge of the protons with their own negative charge, sometimes creating ions if the number of electrons does not equal the number of protons.
An atom with 11 protons, 11 electrons, and 12 neutrons is an atom of sodium.
The element is determined by the number of protons in the nucleus of the atom, and for sodium, this number is 11. Atoms have the same number of electrons as they have protons so the positive charges exactly cancel out the negative charges, resulting in a neutral atom.
State the number of protons, neutrons and electrons in a sodium atom.
Short answer: 11 protons, 12 neutrons, 11 electrons
Long answer: The atomic number tells us the number of protons, so we know that sodium has 11 protons in its nucleus. Atomic numbers are specific to each element and are written at the bottom left hand side of the element’s symbol. Since atoms are neutral, we know then that sodium atoms must also have 11 negative electrons to cancel the charge from 11 positive protons.
The electron arrangement of all atoms can be found in the data booklet.
All the electrons are arranged into energy levels. Each energy level has a set number of electrons it can hold, and this is determined by the number of protons. For example, an atom of sodium has 11 protons, so the first energy level can hold up to 2 electrons, the second can hold up to 8, and the third can hold up to 1. This means that an atom of sodium has 2 electrons in the first energy level, 8 electrons in the second, and 1 electron in the third.
Atoms form chemical bonds in order to become stable.
Atoms want to be stable, and in order to achieve this, they need to have a full outer shell of electrons. The outer shell is also known as the valence shell, and it is the electrons in this shell that are involved in chemical bonding. Atoms can either gain or lose electrons in order to achieve a full outer shell. Sodium, for example, has 1 electron in its valence shell and can easily lose it, forming a positively charged ion.
In conclusion, an atom with 11 protons, 11 electrons, and 12 neutrons is an atom of sodium. It has 11 protons, 12 neutrons, and 11 electrons, and its outer shell can hold up to 2 electrons in the first energy level, 8 electrons in the second, and 1 electron in the third. Sodium atoms form chemical bonds by gaining or losing electrons in order to achieve a full outer shell and become stable.



Leave a Comment