Are you curious to know what element has 23 protons, 21 neutrons, and 23 electrons? Well, you’re in luck, because the answer is Iron. But why is it Iron and not some other element? What is the relation between mass number, number of protons and number of neutrons?
Atoms are made up of three basic particles: protons, neutrons, and electrons. The number of protons in an atom is also known as its atomic number. The atomic number defines an element’s identity and is the same for all atoms of the same element. Neutrons are neutral particles and do not affect an element’s atomic number. The mass number is equal to the number of protons plus the number of neutrons in an atom.
Iron has an atomic number of 26, which means it has 23 protons and 23 electrons. The number of neutrons in an atom of Iron can vary and is known as an isotope. In this case, the isotope of Iron has a mass number of 50, which means it has 27 neutrons. So the combination of the atomic number and the mass number of Iron is 26-50, which means it has 23 protons and 27 neutrons.
So now that you know the answer to the question, “What has 23 protons and 21 neutrons?” You can understand the relationship between the different particles in an atom. It is important to remember that the atomic number is the same for all atoms of an element and the mass number is equal to the number of protons plus the number of neutrons in the atom. Now that you have all the facts, you can be confident in your knowledge of atoms and isotopes.
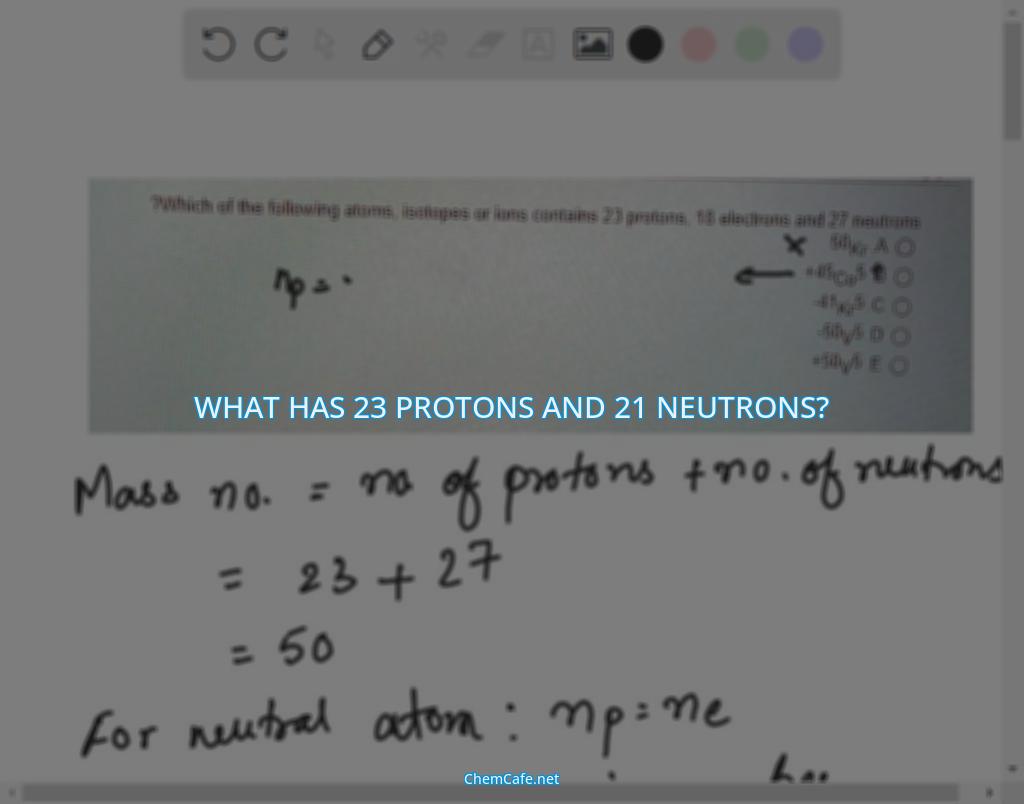
What has 23 protons and 21 neutrons?
Atoms are made up of protons, neutrons, and electrons. The number of protons and electrons in an atom determines its atomic number. So, when we ask what has 23 protons and 21 neutrons, the answer is an isotope of iron.
Atomic Number and Mass Number
Atomic number is the number of protons in an atom and mass number is the total number of protons plus neutrons. So, if the atomic number is 23, then the mass number must be 50 (23 protons + 27 neutrons = 50).
Neutral Atoms
For a neutral atom, the number of protons is equal to the number of electrons. This means that in this case, the number of electrons must also be 23. This is the answer to the question: what has 23 protons and 21 neutrons?
Iron Isotopes
Iron has two naturally occurring isotopes: Iron-56 and Iron-54. Iron-56 has 26 neutrons, while Iron-54 has 24 neutrons. In this problem, the number of protons is 23 and the number of neutrons is 21. This means that the atom in question is an isotope of iron with 23 protons, 18 electrons, and 27 neutrons.
The Relation between Mass Number, Number of Protons, and Number of Neutrons
The mass number of an atom is equal to the number of protons plus the number of neutrons. Knowing this, it is possible to calculate the number of neutrons in an atom. For the isotope of iron in this problem, the number of protons is 23 and the mass number is 50. So, the number of neutrons must be 27 (50 – 23 = 27).
In conclusion, the answer to the question “what has 23 protons and 21 neutrons?” is an isotope of iron with 23 protons, 18 electrons, and 27 neutrons. The mass number is determined by the number of protons plus the number of neutrons, so it is easy to calculate the number of neutrons in an atom when the other two values are known.
What element has 23 protons 23 neutrons and 23 electrons?
The element with 23 protons, 23 neutrons, and 23 electrons is Iron. Iron is a chemical element with symbol Fe and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. Iron has an atomic mass of 55.845 amu and a density of 7.87 g cm−3.
Iron has been known since ancient times and has been used for various purposes throughout history. It is the most abundant element in the Earth’s crust, making up about 5% of the Earth’s mass. Iron is also the most abundant metal in the universe.
Iron is one of the most common elements on Earth and is essential for life. It is essential for the formation of hemoglobin, the oxygen-carrying molecule in red blood cells. Iron is also found in proteins and enzymes, which are important in many biological processes.
The element Iron has 23 protons, 23 neutrons and 23 electrons. This arrangement of particles gives Iron a neutral charge, meaning it has the same number of protons and electrons. The number of protons is equal to the atomic number, which is 26 for Iron. The number of neutrons is equal to the mass number minus the atomic number, which is 27 for Iron.
The relationship between the mass number, the number of protons, and the number of neutrons is known as Soma’s Law. This law states that the mass number is equal to the sum of the number of protons and the number of neutrons. In the case of Iron, the mass number is 50, which is equal to the sum of the number of protons (23) and the number of neutrons (27).
Iron is an atom that has a neutral charge, meaning it has the same number of protons and electrons. This is important because it allows Iron to form bonds with other molecules and helps to keep it chemically stable. Iron can form two types of bonds: covalent and ionic. Covalent bonds involve the sharing of electrons between two atoms, while ionic bonds involve the transfer of electrons from one atom to another.
Iron is a valuable element due to its abundance, low cost, and wide range of uses. It is used in a variety of applications, from making steel to producing magnets. It is also used in medicine, as iron supplements can be used to treat iron deficiency anemia.
In conclusion, Iron is an element with 23 protons, 23 neutrons, and 23 electrons. This arrangement of particles gives Iron a neutral charge, making it chemically stable and allowing it to form bonds with other molecules. Iron is an abundant and inexpensive element, making it an important part of our lives.
What element has 23 protons and 28 neutrons?
The element with 23 protons and 28 neutrons is sodium. Sodium is a chemical element with symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal, and forms a dark oxide-nitride layer when exposed to air. Sodium has an atomic weight of 22.99, meaning it has 11 protons and 12 neutrons for a mass number of 23.
Sodium Atom Structure and Properties
The number of valence electrons of an element has no bearing on its mass number or atomic weight. Sodium has a positive electric charge of +1e and a rest mass equal to 1.67262 × 10−27 kg (938.272 MeV/c2)—marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.
Sodium is a very reactive metal and forms a dark oxide-nitride layer when exposed to air. It also reacts with water to form sodium hydroxide and hydrogen gas. Sodium has an electronegativity of 0.93 and a melting point of 97.8°C. It is a soft metal that melts easily and has a density of 0.968 grams per cubic centimeter.
Protons and Neutrons
The proton has a mean square radius of about 0.87 × 10−15 m, or 0.87 fm, and it is a spin – ½ fermion. It has a positive electric charge and a rest mass equal to 1.67262 × 10−27 kg (938.272 MeV/c2). Protons exist in the nuclei of typical atoms, along with their neutral counterparts, the neutrons.
The neutron has a mean square radius of about 0.8×10−15 m, or 0.8 fm, and it is a spin-½ fermion. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron. Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge.
Uses of Sodium
Sodium is widely used in many industries. It is used in the production of glass, soaps, detergents, and many other products. It is also used as a preservative in food and as an electrolyte in batteries. Sodium is also used in the production of various chemicals, such as sodium hydroxide and sodium bicarbonate.
Sodium is also an essential mineral for the body and is found in many foods, such as fruits, vegetables, and grains. It helps to regulate fluid balance and is important for muscle and nerve function.
In conclusion, the element with 23 protons and 28 neutrons is sodium. Sodium is a soft, silvery-white, highly reactive metal, and forms a dark oxide-nitride layer when exposed to air. It has an atomic weight of 22.99, meaning it has 11 protons and 12 neutrons for a mass number of 23. Sodium is widely used in many industries and is an essential mineral for the body.
Which element has 28 neutrons and 23 protons in its nucleus?
Atoms are made up of protons, neutrons, and electrons. The protons and neutrons make up the nucleus of the atom and the electrons orbit around the nucleus. The number of protons in an atom’s nucleus determines the element. For example, an atom with 6 protons is a Carbon atom. The number of neutrons in an atom’s nucleus can vary, but the number of protons remains the same. This means that an element can have a variety of isotopes, or atoms that have the same number of protons but a different number of neutrons.
So, which element has 28 neutrons and 23 protons in its nucleus? The answer is Vanadium, which is a transition metal located in group 5 of the periodic table. Vanadium has an atomic number of 23, meaning it has 23 protons in its nucleus. It also has 28 neutrons in its nucleus, giving it an atomic weight of 51.
Atomic Structure of Vanadium
Vanadium atoms have a nucleus that consists of 23 protons and 28 neutrons. These are surrounded by 23 electrons arranged in four shells. The electrons in the first shell contain two electrons, the second shell contains eight electrons, the third shell contains thirteen electrons, and the fourth shell contains two electrons.
Chemical Properties of Vanadium
Vanadium is a transition metal, meaning it has properties of both metals and nonmetals. It is a relatively soft metal with a silvery-white color and a high melting point. It is also highly resistant to corrosion, making it ideal for use in a variety of industrial applications. Vanadium is also highly reactive, meaning it can form a variety of compounds when combined with other elements.
Uses of Vanadium
Vanadium is used in a variety of industrial applications, such as steel alloys, cutting tools, and batteries. It is also used in some medical applications, such as an anti-inflammatory drug and an anti-cancer drug. Vanadium is also used to make magnets and other devices that rely on magnetic fields.
In conclusion, Vanadium is an element with 28 neutrons and 23 protons in its nucleus. It is a transition metal located in group 5 of the periodic table and has a variety of industrial and medical uses. Its atomic structure consists of a nucleus with 23 protons and 28 neutrons, surrounded by 23 electrons arranged in four shells.
How many protons and neutrons does vanadium 51 have?
Vanadium 51 is one of the two naturally occurring isotopes of vanadium, the other being vanadium 50. It is composed of 23 protons, 28 neutrons, and 23 electrons. This isotope has a nuclear spin of 7⁄2, which is useful for NMR spectroscopy. It is an important element in the periodic table, with many industrial and scientific uses.
Vanadium Isotopes
Vanadium has several different isotopes, each with its own mass number, atomic number, and number of protons, neutrons, and electrons. The two most common isotopes are vanadium 50 and vanadium 51. Both of these isotopes are stable, meaning they do not undergo radioactive decay.
Vanadium 50 has a mass number of 50, an atomic number of 23, and 23 protons, 27 neutrons, and 23 electrons. Vanadium 51 has a mass number of 51, an atomic number of 23, and 23 protons, 28 neutrons, and 23 electrons.
Uses of Vanadium
Vanadium is used in a variety of industrial and scientific applications. It is a key component in the production of high-strength steel, and is used in the manufacture of magnets, catalysts, and other industrial chemicals. It is also used in the production of alloys, such as titanium-aluminum-vanadium alloys, which are used in the aerospace industry.
Vanadium is also used in scientific research. It is used in NMR spectroscopy to study the structure of molecules, and its nuclear spin is useful for studying the properties of materials. It is also used in the production of radioactive isotopes, which are used in medical imaging and other diagnostic procedures.
Vanadium in the Environment
Vanadium is a naturally occurring element, and is found in small amounts in rocks, soil, and water. It is also released into the environment through the burning of fossil fuels and the production of certain industrial chemicals. However, the concentration of vanadium in the environment is usually very low, and it is not considered a serious environmental pollutant.
Vanadium is an essential trace element for humans, and is needed for proper growth and development. It is found in some foods, such as mushrooms, shellfish, and certain grains. It can also be obtained from dietary supplements.
Vanadium 51 is one of the two naturally occurring isotopes of vanadium, and is composed of 23 protons, 28 neutrons, and 23 electrons. It is an important element in the periodic table, with many industrial and scientific uses. It is also an essential trace element for humans, and is found in some foods and dietary supplements.
What element has a mass number of 51 and 23 electrons?
Atoms are made up of protons, neutrons and electrons, where each of them has specific properties. The element with a mass number of 51 and 23 electrons is Vanadium, which is a chemical element with an atomic number of 23. This means that there are 23 protons and 23 electrons in the atomic structure of Vanadium.
How Does the Atomic Number Determine the Chemical Behavior of Atoms?
The atomic number is an important factor in determining the chemical behavior of atoms, as it is the number of protons present in the nucleus. The number of protons determines the element, and the number of electrons determines the chemical behavior. This is because the electrons are responsible for the chemical behavior of atoms.
For example, Hydrogen has an atomic number of 1, which means that there is one proton and one electron in the nucleus. The electron configuration of Hydrogen is written as:
H: 1s1
This means that the single electron completely fills the first energy level.
Atomic Mass of Vanadium
The atomic mass of Vanadium is 50.9415 u. Note that each element may contain more isotopes, which are atoms of the same element with a different number of neutrons.
Vanadium is a transition metal, and its elemental properties are determined by the number of electrons present in its outermost shell. Since Vanadium has an atomic number of 23, it has 23 electrons. This means that a neutral Vanadium atom has 23 electrons.
Electron Configuration of Vanadium
The electron configuration of Vanadium is written as:
V: [Ar] 3d3 4s2
This means that the Vanadium atom has two electrons in the 4s orbital, and three electrons in the 3d orbital. This electron configuration gives Vanadium its unique properties, such as its high melting point, and its low reactivity.
In conclusion, the element with a mass number of 51 and 23 electrons is Vanadium. The atomic number of Vanadium is 23, which means that there are 23 protons and 23 electrons in the atomic structure. The atomic mass of Vanadium is 50.9415 u, and its electron configuration is written as [Ar] 3d3 4s2. This electron configuration gives Vanadium its unique properties.



Leave a Comment