What Chemical Is Formed When Isopropyl Alcohol and Vinegar Are Mixed?

Mixing isopropyl alcohol (IPA) and vinegar mainly produces a physical mixture, with only a small amount of isopropyl acetate formed via esterification. The main components remain largely unchanged, as no significant chemical reaction occurs under normal conditions.
Composition of the Mixture
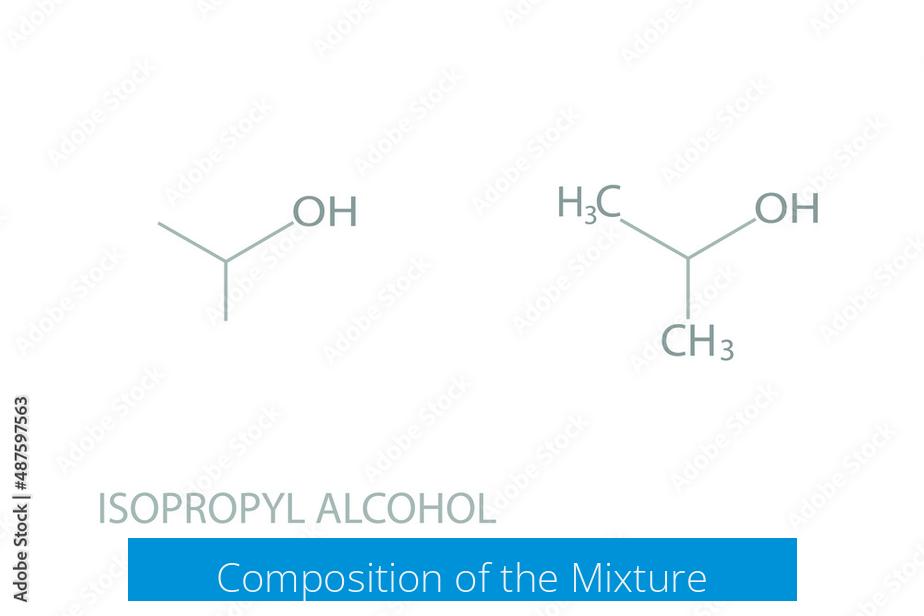
The mixture mostly consists of isopropyl alcohol and acetic acid, which is the active ingredient in vinegar. Both substances coexist without forming a new major compound. About 99% of the mixture consists of these original chemicals.
Potential Chemical Reaction
- Isopropyl alcohol can react with acetic acid in an esterification reaction.
- This reaction produces isopropyl acetate, a common ester known for fruity odors.
- However, this esterification is very limited without controlled conditions such as heating, catalysts, or water removal.
- In typical room temperature mixing, the amount of isopropyl acetate formed is negligible.
Practical Considerations for Storage
Since the mixture holds both isopropyl alcohol and acetic acid, the choice of storage container should consider compatibility with both chemicals to prevent degradation.
- Materials suitable for storing either isopropyl alcohol or acetic acid individually often suffice.
- The minor presence of isopropyl acetate does not usually require special storage provisions.
Summary of Key Points
- The mixture is largely a physical combination rather than a new chemical entity.
- Isopropyl acetate may form in trace amounts via esterification but is not the main product.
- Controlled conditions are needed to produce significant isopropyl acetate.
- Storage materials should be compatible with both isopropyl alcohol and acetic acid.
For further information about the possible ester formed, readers may consult the isopropyl acetate entry on Wikipedia.
What happens chemically when isopropyl alcohol and vinegar are mixed?
The mixture is mostly physical. Both remain largely unchanged. However, a small amount of isopropyl acetate can form through esterification.
What is isopropyl acetate in the mixture?
It is a minor product formed when isopropyl alcohol reacts with acetic acid from vinegar. The reaction needs specific conditions to produce significant amounts.
Does mixing isopropyl alcohol and vinegar create a new compound?
No major new compound forms under normal conditions. The mixture remains mainly two separate chemicals with only tiny conversion to isopropyl acetate.
How should the mixture of isopropyl alcohol and vinegar be stored?
Storage should suit both isopropyl alcohol and acetic acid. The small amount of isopropyl acetate generally does not affect container choice.
Can isopropyl alcohol and vinegar mixture be used as a cleaner?
Yes, their combined properties help clean surfaces. But the mixture mostly behaves as a physical blend with no enhanced chemical effects.




Leave a Comment