What Does 1F Mean?
1F means one formula weight per liter, a unit synonymous with formal concentration, also called formality. In chemistry, 1F denotes 1 formula weight of a substance dissolved in one liter of solution and is often used interchangeably with molarity.
Definition of 1F
1F stands for one formula weight per liter of solution. Linus Pauling, a notable chemist, uses 1F interchangeably with molar concentration in his book General Chemistry. This usage indicates that 1F equals 1 mole of a substance dissolved per liter.
In practical terms, 1F simply means 1 formula weight unit of a compound dissolved in exactly one liter of solution. It’s a measure of concentration, expressing the total amount of a substance regardless of its chemical form in the solution.
Formality (F) vs. Molarity (M)
Molarity Explained
Molarity (M) describes the concentration of a particular chemical species as moles of solute per liter of solution. For substances that do not dissociate in water, molarity directly corresponds to the number of moles present.
- For example, 0.0259 moles of glucose dissolved in 1 liter solution results in 0.0259 M glucose, because glucose remains intact without dissociation.
- In contrast, calcium chloride (CaCl2) dissolves and dissociates into ions. If 0.1 moles of CaCl2 dissolve in 1 liter of water, the molarity of CaCl2 itself is effectively 0 M. Instead, the solution contains 0.1 M Ca2+ ions and 0.2 M Cl- ions.
Formality Explained
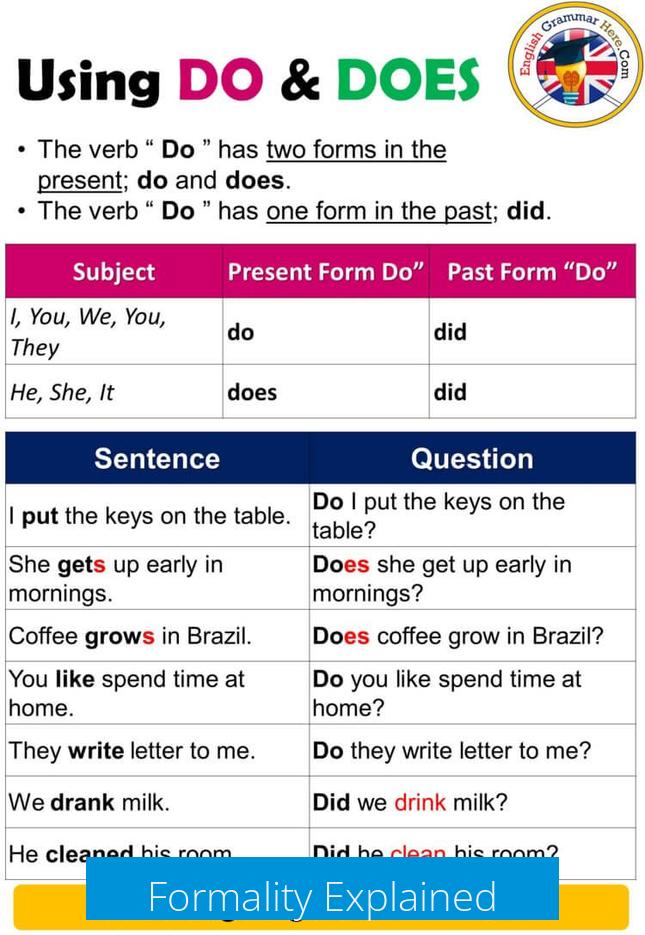
Formality (F) is the total concentration of the chemical substance, measured as formula weights per liter, without considering whether or not the substance dissociates.
- For compounds like glucose, formality equals molarity because the substance does not dissociate; thus, 0.0259 moles per liter is 0.0259F and 0.0259M.
- For dissociating compounds like CaCl2, formality indicates the total amount of CaCl2 present prior to dissociation. Therefore, 0.1 moles dissolved result in 0.1F CaCl2, even though molarity of intact CaCl2 is 0.
Practical Use and Confusion
In practice, formality and molarity often get used interchangeably because both quantify moles per liter. They are technically different when dealing with substances that dissociate, but many texts and professionals treat them as equivalent.
This interchange causes confusion. Searches for “1F” as a distinct term may yield few results, and some speculate that it may be shorthand specific to particular authors or regions. Nevertheless, chemists understand 1F as a concentration unit akin to molar.
Other Meanings of “1F” Outside Chemistry
1F Marked in Snow: Social Media Case
Recently, “1F” appeared written in snow atop a trash bin, documented in a popular TikTok video by user Jade Jules. The video asked viewers what “1F” meant, gaining over 11 million views.
Initial Reactions and Theories

Many viewers feared that “1F” could be a warning or marker left by criminal groups, such as sex traffickers or kidnappers. The theory suggested “1F” meant “one female” tagged at a location. This drew significant attention and concern in the comments.
Follow-Up and Law Enforcement Response
The TikTok creator called police regarding the marking. Authorities reportedly investigated the house across the street. However, no conclusive action or explanation emerged. The user later shared that she stayed with family due to safety concerns.
Debunking and Skepticism
Similar claims about “1F” as a trafficker’s code have been debunked by fact-checking organizations like Snopes. Past rumors about markings like “1F” or “1C” on cars or houses were proved false, with no law enforcement verification.
TikTok commentators who investigate misinformation highlighted that such videos often spread false alarm. Some accounts spreading these theories have histories of promoting unverified claims, intensifying public confusion.
Summary of 1F Meanings
| Context | Meaning of 1F | Explanation |
|---|---|---|
| Chemistry | 1 formula weight per liter (1F) | The total concentration of a substance expressed as formula weights dissolved in one liter of solution; synonymous with formal concentration. |
| Social Media / Public Concern | Unknown / Alleged marker | Appears as graffiti or marks; rumored as code by traffickers (“one female”), but these claims lack evidence and are debunked. |
Key Takeaways

- 1F in chemistry stands for 1 formula weight dissolved per liter and equals formality, often used like molarity.
- Formality accounts for total chemical concentration, ignoring dissociation; molarity measures concentration of chemical species present.
- For non-dissociating compounds, molarity and formality are the same; they differ for ionic compounds.
- The “1F” marking spotted in snow triggered unfounded fears linked to human trafficking codes but was debunked by authorities.
- Skepticism is essential when interpreting unusual markings or viral social media claims to avoid spreading misinformation.
What does 1F mean? Unlocking the Mystery Behind the Term
If you’ve come across ‘1F’ and wondered what it means, you’re not alone. 1F stands for ‘formula weight per liter,’ which is essentially the same as molarity (moles per liter) in chemistry. It’s a measure of concentration indicating how many formula weights of a substance are dissolved in one liter of solution. But wait, there’s more to the story—so buckle up as we dive into chemistry, TikTok mysteries, and why ‘1F’ might mean very different things depending on where you find it.
‘1F’ in the World of Chemistry: What It Really Means
In chemical terms, ‘1F’ is a shorthand for “formality,” a concept that sometimes gets mixed up with “molarity.” But aren’t they the same? Well, kind of.
Formality (symbol F) refers to the total concentration of a substance in solution, ignoring how it may break apart. For example, if you dissolve 0.1 moles of calcium chloride (CaCl2) in one liter of water, you have a formality of 0.1 F CaCl2. However, because CaCl2 dissociates into ions (Ca2+ and Cl-), its molarity with respect to the intact compound is effectively zero. Instead, you get concentrations of 0.1 M Ca2+ and 0.2 M Cl-.
On the other hand, molarity (symbol M) specifies the concentration of the solute species actually present in the solution. It’s a measure based on moles of solute per liter of solution. For substances that don’t dissociate, like glucose, molarity and formality are the same. Dissolve 0.0259 moles of glucose in one liter, and you have 0.0259 M or 0.0259 F glucose.
Linus Pauling, a Nobel laureate and chemistry legend, even uses “formality” and “molarity” interchangeably in his General Chemistry book. That’s a good sign this confusion isn’t new! In short, 1F means 1 formula weight per liter—just like 1 Molar.
Why Does the Confusion Between Formality and Molarity Matter?
Because the way substances behave in solution varies, relying solely on molarity can sometimes be misleading. For salts that dissociate, such as CaCl2, molarity measures the ions present, not the original compound. Formality, conversely, counts the total formula units dissolved regardless of dissociation.
This sounds subtle but is crucial in lab work and chemical calculations. If you’re mixing solutions or calculating reaction stoichiometry, knowing whether to apply molarity or formality ensures accuracy. Remember, if your solute doesn’t break apart, 1F and 1M are practically twins.
When ‘1F’ Has Nothing to Do with Chemistry: The TikTok Snow Mystery

Now, let’s pivot to a very different and far less scientific context where ‘1F’ caused a social media sensation. On January 12, TikTok user Jade Jules posted a viral video showing ‘1F’ written in snow atop her trash bin with the caption “What the fuck does this mean?” The video exploded with over 11 million views.
Some viewers panicked. Theories popped up suggesting that ‘1F’ was a mark left by sex traffickers or kidnappers signaling “one female” at the house—startling stuff! Commenters debated wildly, spreading fear. Jade even called the police, who responded by visiting a nearby house, but no concrete evidence emerged, and the situation remains murky.
The Truth Behind 1F Markings: Misinformation and Hoaxes
This story fits a pattern we’ve seen before. Last summer, social media rumors swirled about human traffickers marking cars with codes like ‘1F’ or ‘1C’ (one child). Local law enforcement in Humboldt County and elsewhere debunked these claims.
Fact-checkers like Snopes have labeled these kinds of posts as misinformation. Skeptics point out that prominent TikTok accounts spreading such videos often also share misleading content. The ‘1F’ snow marking appears to be another example where fear and misunderstanding run rampant.
That’s why awareness is critical—but so is careful fact-checking. Viral videos can create waves powerful enough to stir panic but sometimes have little factual footing.
Wrapping Up: So, What Does 1F Mean?
Depending on context, ‘1F’ either:
- In Chemistry: Means one formula weight per liter, essentially showing total solute dissolved without considering dissociation. It’s mostly synonymous with molarity, especially for substances that do not dissociate in solution.
- On Social Media Snow Markings: Has become a symbol clouded by rumors and fear, often misunderstood as a secret code related to dangerous activities—but officially debunked as unsubstantiated.
So, before you freak out about mysterious ‘1F’ markings—whether in your chemistry class or on your trash bin—take a breath and check the facts.
Do you work with chemical solutions? Next time you see ‘1F’ in a lab report, remember it refers to formality, a handy way to describe concentration.
Encounter ‘1F’ outside science? Ask questions and seek reliable info before jumping to conclusions.
Bonus Chemical Tip: How to Remember Formality vs Molarity
Think of molarity as what’s really swimming in your solution—the ions and molecules actually present. Formality is the amount you started with, counting everything you dumped in, point blank.
In cases where compounds don’t split up, molarity and formality walk hand-in-hand. When they dissociate, molarity gets more specific, formalities stay general.
Final Thought: The Power of Context
Isn’t language fun? A tiny phrase like ‘1F’ can mean a precise scientific measurement—or fuel a viral mystery that grips millions. Context is king. Always dig deeper and ask: “What does 1F mean here?”
And if you’re ever stuck, just imagine Linus Pauling nodding sagely in the background, saying, “It’s just molarity, folks.”
What does 1F mean in chemistry?
1F stands for one formula weight per liter, which is the same as molarity. It measures concentration as moles of a substance in one liter of solution.
How is 1F different from molarity?
1F, or formality, counts the total concentration without considering dissociation. Molarity counts actual moles of dissolved species, accounting for ionization in solution.
Why is 1F used interchangeably with molarity?
They both express concentration as moles per liter. For non-dissociating compounds, 1F equals molarity. The terms differ mainly for substances that dissociate.
What does 1F mean when marked in snow or on property?
“1F” in such contexts sparked fears it signals a female target for criminals. However, law enforcement and fact-checkers have found no proof supporting this claim.
Is the “1F” marking related to kidnapping or trafficking?
Claims connecting “1F” to human trafficking are unverified and debunked. These markings appear to be misinformation spread on social media platforms.
Why did the 1F snow marking video go viral on TikTok?
People reacted with fear and shared theories. Some accounts linked it to trafficking conspiracies, but experts warn it fuels false information and panic.




Leave a Comment