What Does Balancing an Equation Actually Do/Mean?
Balancing a chemical equation establishes the precise proportional relationship between reactants and products to uphold the law of conservation of mass. It defines the ratios needed for reactants to fully convert to products, facilitating quantitative stoichiometric calculations and reaction optimization.
Ensuring Mass Conservation and Defining Ratios
Chemical equations must be balanced to obey the law of conservation of mass. This principle states that matter cannot be created or destroyed in a chemical reaction. Balancing the equation adjusts the coefficients so that the total number of atoms of each element is equal on both sides.
Balanced equations specify the ratio in which substances react, not the exact amounts used in every scenario. For example, consider the reaction:
| Unbalanced | Balanced |
|---|---|
| 2A + B → BA2 | 2A + B → BA2 |
Here, 2 parts of A react with 1 part of B. If you have 30 grams of A, the stoichiometric ratio implies it will react completely with 15 grams of B. If only 10 grams of B are available, only 20 grams of A will react, leaving 10 grams of A unreacted.
Providing Stoichiometric Ratios for Calculations and Optimization
Balancing equations supports stoichiometry—the calculation of reactant and product quantities in chemical reactions. Knowing the exact mole ratios allows chemists to:
- Predict the theoretical yield of products.
- Determine the limiting reagent controlling reaction extent.
- Optimize reagent amounts to reduce waste and improve efficiency.
Although a reaction can proceed without balancing the chemical equation, unbalanced equations lack the quantitative relationships necessary for these calculations. Optimization of reactions relies on accurate stoichiometric data to understand how much of each reactant is required.
Reactions often involve kinetic steps and rate-determining steps, where the balanced equation ensures precise measurement of inputs and predicted outputs, which is critical in designing industrial processes or laboratory syntheses.
Balanced Equations Represent Ideal Ratios, While Real Reactions May Vary
Balanced chemical equations communicate ideal reaction ratios that achieve complete consumption of reactants without leftovers. However, in practical settings, reactions can occur whenever reactants encounter each other, even if amounts do not perfectly match.
Excess reagents remain unreacted in such cases, and reaction conditions such as temperature, pressure, and catalysts influence outcomes. Chemists often intentionally use an excess of a reagent to drive reactions toward completion and maximize product yield. For example:
To make 12 pancakes, you need 100 g flour, 2 eggs, and 300 mL milk — this combination is similar to a balanced chemical equation. If the amounts vary, you can still make pancakes but will have leftover ingredients.
Similarly, chemical reactions can proceed even with unbalanced inputs but will result in leftover substances and less efficient yield.
Implications of Balancing Beyond Simple Atom Counting
Balancing goes beyond just matching atoms on either side. It enables:
- Quantitative predictions of product amounts from given reactants.
- Identification of limiting reagents, critical for calculating maximum possible yields.
- Adjustment of feed ratios in industrial processes to optimize costs and reduce waste.
Without balancing, these calculations become inaccurate, and optimizing reaction conditions is impossible.
Key Points to Remember
- Balancing reflects the conservation of mass by accounting for every atom in reactants and products.
- It establishes precise ratios, not absolute quantities, enabling stoichiometric calculations.
- Balanced equations are essential for predicting theoretical yields and optimizing reagent amounts.
- Reactions still occur when ratios are uneven, but excess reactants remain unconsumed.
- Real reactions depend on additional factors like reaction conditions and kinetics.
What Does Balancing an Equation Actually Do/Mean?

Let’s start with the straightforward answer: balancing an equation ensures the reactants and products obey the law of conservation of mass by defining the exact ratios in which substances react and form products. It means you know how much of one substance will react with another under ideal, perfect conditions. But there’s way more to it than just matching numbers on paper!
Think of balancing chemical equations as the blueprint for understanding chemical reactions in a precise way. It’s the difference between guessing and calculating the exact amount of materials needed and what you can expect to get out of a reaction.
Mass Conservation and Ratio Representation: The Backbone of Balancing
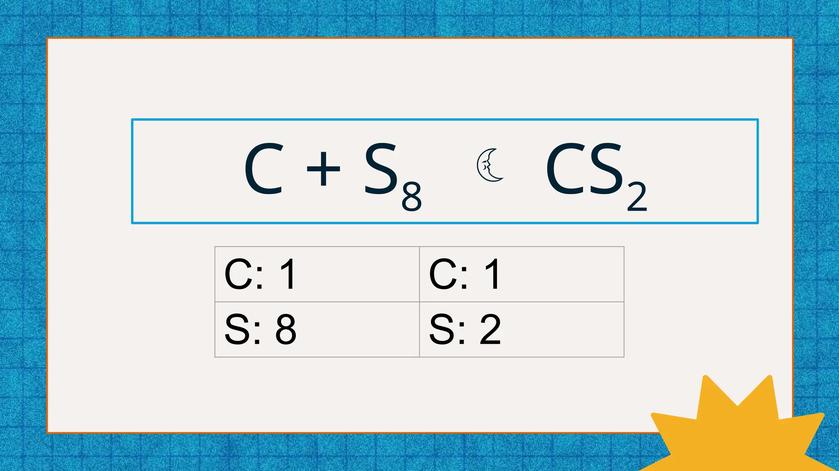
First and foremost, balancing an equation is about mass conservation. You cannot make or lose atoms in a reaction; these tiny bits don’t just disappear into thin air. Imagine you have a reaction represented by:
2A + B → BA2
This balanced equation tells you 2 parts of A combine with 1 part of B to make a product. It’s not just a random number game or a puzzle—it’s a direct reflection of how atoms actually pair up.
For example, if you have 30 grams of A, to fully react without leftovers, you need 15 grams of B. This is the stoichiometric ratio in action. Suppose you only provide 10 grams of B. Then, only 20 grams of A will react, leaving 10 grams of A unreacted, or in excess. That’s the real-world impact of not having perfectly matched amounts. Your leftover stuff is not magical; it’s just chemistry telling you “Hey, you didn’t bring enough B to the party!”
The Real Deal: Stoichiometric Ratios for Calculations and Optimization
In the chemistry kitchen, knowing these ratios is essential. Why? Because if you don’t balance your equation, you’re basically blindfolded in the kitchen trying to whip up pancakes without knowing how much flour or eggs to use.
Balancing gives you access to stoichiometry, the tool chemists use to calculate the exact amounts of reactants needed and the theoretical yield of products. You want to figure out how much product you can expect if everything goes perfectly? Balancing is your first stop.
Without it, you’d struggle to optimize your reaction. Optimization includes adjusting reaction conditions and reactant proportions to maximize product while minimizing waste.
A balanced equation isn’t just a mental exercise, it’s your go-to for planning lab experiments and industrial chemical processes. It helps you know the input, output, and waste, saving time, money, and headaches.
Balanced Equations: Ideal Ratios and Real-World Reactions
Balanced equations show an ideal. But here’s a little chemistry nugget: the reaction will still occur whenever molecules meet, even if you’re not hitting those perfect ratios.
What happens then? You get leftovers—the stuff that didn’t get used up because the other reactant ran out. It’s like trying to bake pancakes but bringing 100 grams of flour and only 1 egg instead of 2. You’ll still get pancakes but not as many as the recipe says, and you’ll have spare flour sitting frustrated in the cupboard.
In actual chemical syntheses, chemists often add a slight excess of one reactant on purpose to push the reaction forward and increase yield. This strategic imbalance contrasts with the balanced equation, which represents the “ideal,” not always the practical.
Moreover, real reactions depend on conditions like temperature, pressure, catalysts, and equilibrium states. These factors can tweak appearances and outcomes beyond what stoichiometry alone predicts. Think of these as the spices and cooking environment that influence your final dish.
Summary: What Balancing an Equation Really Means
Balancing a chemical equation is about setting the right proportional ratios so the law of conservation of mass holds true. It creates a reliable framework for calculating the exact quantities of reactants and products. This precision allows chemists to predict theoretical yields and optimize chemical processes effectively.
But balancing isn’t about deciding if a reaction will occur. Instead, it describes how substances ideally combine if they do react. It’s the chemical handshake choreography, ensuring every atom moves in sync.
Why Should You Care? Practical Tips and Takeaways
- If you ever want to know your theoretical yield—that is, the maximum product possible—start with a balanced equation.
- Planning reaction scale-up for industry? Balancing helps predict costs and waste.
- Limiting reagents: Original balanced ratios identify the reagent that runs out first, helping diagnose and fix yield problems.
- Don’t be afraid to tweak reactants in practice, but always keep balanced equations as your reference point.
- In education, mastering balancing skills builds a solid foundation for understanding chemical behavior and stoichiometry.
Final Thought
Balancing equations may feel like a chore, but it’s the chemistry version of finding a recipe’s perfect proportions. Skip it, and you cook blind. Nail it, and you unlock the secrets to efficient and predictable chemical reactions. So next time you see a sea of letters and numbers, remember: it’s not just math—it’s the key to making chemistry work.
What does balancing a chemical equation actually ensure?
Balancing ensures mass conservation. It defines the exact ratio in which reactants combine and products form. This follows the law of conservation of mass.
Does balancing an equation tell you the amount of substances used?
No, it gives the ratio in which substances react. You use this ratio to calculate actual amounts based on available material.
Why is balancing equations important for chemical calculations?
It provides stoichiometric ratios needed to find theoretical yields and optimize reactions. Without balancing, you cannot accurately measure inputs or outputs.
Can reactions happen if the equation is unbalanced?
Yes, reactions still occur whenever reactants meet. But leftover excess reactants remain, and the predicted product amounts may be incorrect.
How do real-world conditions affect the meaning of a balanced equation?
Balanced equations show ideal ratios but real reactions depend on conditions like equilibrium and kinetics. Excess reagents may be added to improve yields.




Leave a Comment