Differences Between Regioselective, Regiospecific, Stereospecific, and Stereoselective Reactions
Regioselective, regiospecific, stereospecific, and stereoselective describe how chemical reactions favor or restrict the formation of certain isomers in products. They differ by how strict the reaction mechanism is in controlling product formation and whether it concerns positional (constitutional) isomers or spatial (stereoisomer) arrangements.
Regiospecific vs Regioselective

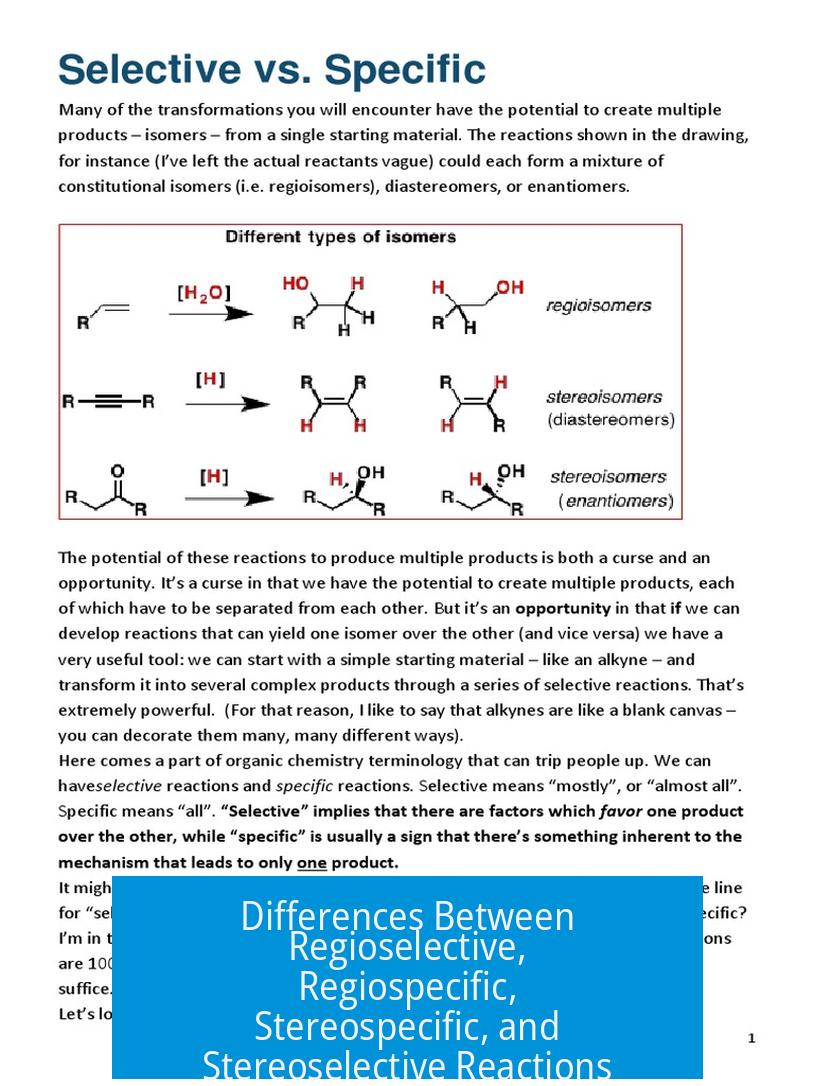
Regiospecific reactions only produce one constitutional isomer. The reaction mechanism permits a single positional outcome without alternatives. For example, Markovnikov addition to alkenes strictly yields one regioisomer where the electrophile bonds to the more substituted carbon.
Regioselective reactions produce multiple constitutional isomers but favor one preferentially. The outcome is not exclusive, but one regioisomer dominates due to kinetic or thermodynamic factors. An example is E1 and E2 eliminations that favor the more substituted alkene but still generate minor amounts of less substituted isomers.

Stereospecific vs Stereoselective
Stereospecific reactions yield only one stereoisomer by mechanism. The pathway enforces a strict stereochemical outcome. A classic example is the SN2 reaction, which universally inverts stereochemistry at the reactive carbon, producing one specific stereoisomer.
Stereoselective reactions can form several stereoisomers but prefer one. Multiple stereoisomers are possible, but one forms in greater amounts due to lower energy or faster formation. For instance, E2 eliminations tend to favor trans alkenes but can produce cis isomers as minor products.
Summary Table
| Term | Products Allowed | Outcome Control/Mechanism | Example |
|---|---|---|---|
| Stereospecific | One stereoisomer only | Mechanism allows only one stereochemical outcome | SN2 inversion of configuration |
| Stereoselective | Multiple stereoisomers, one favored | Multiple pathways; one energetically preferred | E2 elimination favoring trans alkene |
| Regiospecific | One constitutional isomer only | Mechanism permits only one positional outcome | Markovnikov addition to alkene |
| Regioselective | Multiple constitutional isomers, one favored | Multiple possibilities; one preferred thermodynamically or kinetically | E1/E2 elimination forming more substituted alkene |
Clarifying Specificity Versus Selectivity
A reaction can be 100% stereoselective or regioselective without being purely specific.
- Specificity means the mechanism excludes all alternatives.
- Selectivity means the reaction allows alternatives but favors one.
Example: An SN2 reaction is stereospecific since inversion is the only outcome. An E2 elimination is stereoselective, producing mostly one stereoisomer but minor amounts of others remain possible.
Key Takeaways
- Regiospecific and stereospecific reactions produce only one constitutional or stereoisomer, respectively.
- Regioselective and stereoselective reactions produce multiple isomers but favor one strongly.
- Specificity implies an exclusive mechanism; selectivity implies preferential formation.
- Understanding these terms clarifies reaction outcome predictions in organic synthesis.
What Exactly Is the Difference Between Regioselective, Regiospecific, Stereospecific, and Stereoselective?
Ah, the fascinating world of organic chemistry! It’s packed with complex terms like *regioselective*, *regiospecific*, *stereospecific*, and *stereoselective*. They sound like variations of a spelling bee contestant’s repertoire, yet each one carries a distinct meaning. So, what exactly is the difference between regioselective, regiospecific, stereospecific, and stereoselective reactions? Simply put: the terms describe how reactions control which exact form of a product appears, focusing on location (regio) and spatial arrangement (stereo) of atoms.
Let’s break down these terms step-by-step with a fresh lens—and maybe a dash of humor.
The Basics: Location vs. Orientation
First off, “regio” stems from the word “region,” which here means *where* a reaction takes place on a molecule. Meanwhile, “stereo” relates to *how* the atoms arrange themselves in three-dimensional space. These two perspectives set the stage for understanding the differences.
Stereospecific: The Only Way to Get One Stereoisomer
Stereospecific reactions are like those strict event planners who only allow *one* type of guest—no plus ones, no exceptions. If the reaction happens, there’s only one stereochemical result possible. This is because the reaction mechanism (the chemical choreography) demands it.
Take the SN2 reaction: it strictly leads to an inversion of stereochemistry. No other stereoisomers bake in the reaction oven. So, the outcome isn’t just favored—it’s the *only* possibility. This singular result defines stereospecificity.
Stereoselective: More Choices, But a Clear Favorite
Now, stereoselective reactions are a bit like a party with many guests—more than one stereoisomer can show up, but one tends to dominate the scene. The reaction slants toward producing one stereoisomer more than the others, thanks to factors like energy preferences or kinetics.
Imagine the E2 elimination reaction. It doesn’t deliver a single exclusive product but usually favors the formation of the trans isomer. The mechanism permits multiple stereoisomers, but one product steals the spotlight. This is stereoselectivity in action—more democratic than stereospecific, but with a clear favorite.
Regiospecific: The One-and-Only Constitutional Isomer
Switching gears to “regio”: in regiospecific reactions, the mechanism guarantees a single product based on *where* the reaction occurs on a molecule. This is strict specificity in the territory of structural isomers.
Here, think about Markovnikov’s addition to alkenes. The reaction exclusively produces one constitutional isomer (where the functional group attaches to the more substituted carbon), with no alternative isomer possible. It’s like the GPS of chemistry pointing to a single precise spot.
Regioselective: When Multiple Isomers Are Possible, But One Rules
Regioselective reactions allow more than one structural isomer as a potential product. However, one tends to dominate because of speed (kinetics) or stability (thermodynamics). It’s a preference, not a rule.
For example, E1 or E2 eliminations might yield several alkene isomers, but the product with the more substituted double bond often prevails. Multiple isomers could walk through the door, but the “best dressed” is favored.
Selective vs. Specific: Clarifying the Confusion
A common misunderstanding is to treat selectivity and specificity as interchangeable. They’re not. You can have 100% stereo- or regioselectivity without achieving specificity.
A reaction is specific if the mechanism permits only one outcome—no alternatives allowed. In contrast, it is selective if multiple outcomes are possible, but one is preferred due to energy, kinetics, or other factors.
So, a reaction can be completely regioselective—producing one dominant isomer while in theory others can form—without being regiospecific if the mechanism doesn’t preclude those alternatives.
Summary Table: Quick Comparison
| Term | Products Allowed | Outcome Control/Mechanism | Example |
|---|---|---|---|
| Stereospecific | Only one stereoisomer | Mechanism allows only one stereoisomer | SN2 reaction (inversion only) |
| Stereoselective | More than one stereoisomer | Mechanism allows multiple; one preferred | E2 elimination (more trans formed) |
| Regiospecific | Only one constitutional isomer | Mechanism allows only one constitutional isomer | Markovnikov addition |
| Regioselective | More than one constitutional isomer | Mechanism allows multiple; one preferred | E1 or E2 (more substituted alkene) |
Bringing It Home: Why Should You Care?
Understanding these nuances is critical when predicting reaction outcomes or designing synthesis pathways. Imagine you’re crafting a complex drug molecule; knowing whether a reaction is stereospecific or just stereoselective shapes your strategy and expectations.
Also, this knowledge saves you from the frustration of ambiguous results. It helps answer questions like: Will I get a pure stereoisomer, or a mixture? Can I trust the reaction to only hit one spot on my molecule, or should I prepare for side products?
Practical Tips to Remember
- Ask: Is there only one possible product allowed mechanistically? If yes, it’s specific (stereo- or regio-).
- If multiple products can appear but one dominates, you’re dealing with selectivity, not specificity.
- SN2 reactions are your go-to examples of stereospecific processes—predictable and direct.
- Markovnikov’s addition is the regiospecific star—only one constitutional isomer emerges.
- Reactions like E2 and E1 eliminations often show selectivity, favoring the more stable or substituted isomer.
Don’t worry if it sounds tricky at first. The more you apply these concepts, the clearer they become. It’s like learning the rules of a new game—once you get it, you can start winning!
Final Thought
So next time someone drops “regioselective” or “stereospecific” in a chemistry conversation, you’ll know these aren’t just fancy words. They’re the subtle instructions governing how molecules behave under reaction conditions. And understanding those instructions transforms chemistry from random fireworks into a precise craft.
What is the main difference between regioselective and regiospecific reactions?
Regiospecific reactions produce only one constitutional isomer due to their mechanism. Regioselective reactions can form multiple isomers but favor one over others.
How does stereospecificity differ from stereoselectivity?
Stereospecific reactions yield only one stereoisomer based on their mechanism. Stereoselective reactions can form several stereoisomers but prefer one, often for energetic reasons.
Can a reaction be 100% stereoselective but not stereospecific?
Yes. If the mechanism allows multiple stereoisomers but one dominates fully, the reaction is stereoselective, not stereospecific.
Why is the SN2 reaction considered stereospecific?
Because it always produces inversion of stereochemistry, allowing only one stereoisomer as the product.
In what situation would a reaction be called regioselective?
When it can form multiple constitutional isomers but prefers producing one dominant product, like E1 or E2 eliminations favoring the more substituted alkene.




Leave a Comment