Understanding Atoms and Molecules
What Is an Atom?
An atom is the smallest particle characteristic of a chemical element. It retains the properties of that element. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons. Each element’s atoms have a unique number of protons, called the atomic number, which defines its identity.
What Is a Molecule?
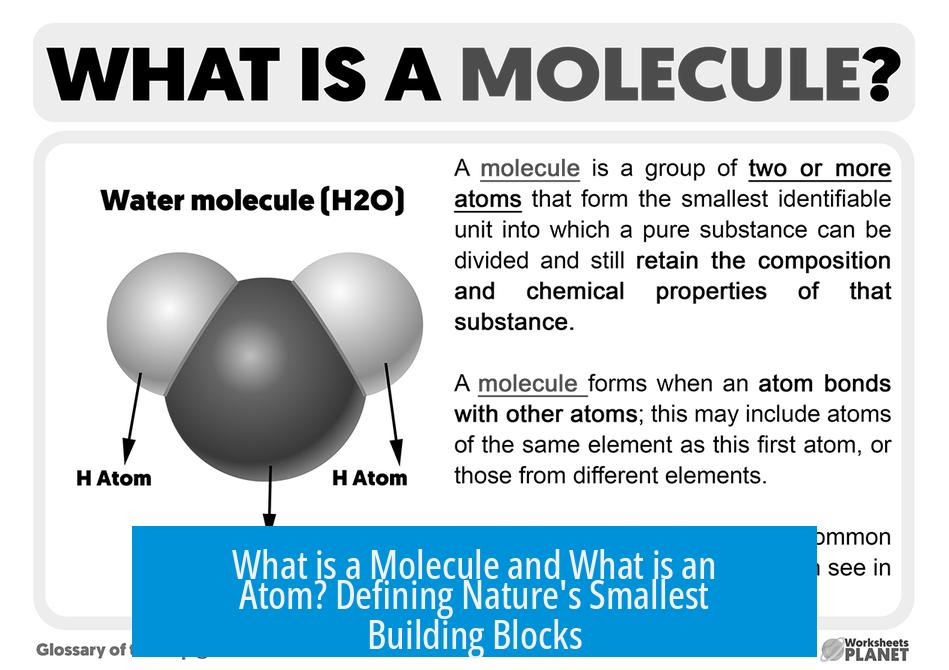
A molecule is a discrete group of two or more atoms chemically bonded together in exact numbers defined by a chemical formula. Molecules form when atoms share or transfer electrons, creating stable structures. These atoms can be the same or different elements.
Types of Molecules
- Molecules as Elements: Consist of two or more atoms of the same kind chemically bonded.Example: Oxygen gas (O2) consists of two oxygen atoms bonded together. Sulfur (S8) forms molecules of eight sulfur atoms.
- Molecules as Compounds: Contain atoms of different elements bonded together.Example: Water (H2O) has two hydrogen atoms and one oxygen atom bonded. Glucose (C6H12O6) includes carbon, hydrogen, and oxygen in fixed proportions.
Distinguishing Molecules from Other Chemical Structures
Not all compounds exist as discrete molecules. Some form extensive structures without distinct molecular units.
| Compound Type | Structure | Example | Characteristic |
|---|---|---|---|
| Ionic Compounds | Lattice Structure | NaCl (table salt) | Positive and negative ions arranged in a repeating pattern |
| Covalently Bonded Networks | Extended 3D Networks | SiO2 (quartz) | Atoms connected in continuous networks, no discrete molecules |
In such compounds, the chemical formula shows the ratio of atoms or ions but does not represent distinct molecules.
Summary
- An atom is the smallest particle defining a chemical element.
- A molecule is a distinct group of two or more atoms bonded chemically in a fixed ratio.
- Molecules can contain atoms of the same element or different elements.
- Some compounds do not form molecules but exist as ionic lattices or covalent networks.
What is a Molecule and What is an Atom? Unpacking the Tiny Building Blocks of Everything
At their core, an atom is the smallest particle that still fully represents a chemical element, while a molecule is a group of two or more atoms chemically bonded together, forming a distinct unit defined by a chemical formula. This simple distinction opens the door to understanding the universe around us—from water we drink to the air we breathe.
Let’s take a deep dive into these tiny marvels and unravel their differences, types, and the fascinating structures they form. Along the way, we’ll pepper in some engaging examples and helpful clarifications that make science feel less daunting and more like an intriguing mystery ready to be solved.
Atoms: The Lone Wanderers of the Chemical World
An atom is basically the smallest piece of an element that still behaves like that element. Imagine a Lego brick that can’t be broken down further without losing the color or shape that defines it. That’s your atom. Each atom carries the identity of its element, like hydrogen or oxygen, by the number of protons in its nucleus.
Atoms aren’t usually loners, though—they love to team up. But before we talk about that, keep in mind this: the atom is the fundamental building block. It’s what chemists and scientists start with in understanding how matter is constructed.
Molecules: When Atoms Join Forces
So what happens when atoms decide to hang out together? They form molecules. A molecule is a discrete group of two or more atoms chemically bonded together. The key word here is “discrete” – molecules are individual, distinct entities—not an endless network.
For example, the molecule O2 (oxygen gas) consists of two oxygen atoms bonded tightly. This is a molecule made of atoms of the same element – a molecule as an element. Another example is S8, a molecule made of eight sulfur atoms. Both are molecules formed by repeating a single atom type.
Then there are molecules made from different kinds of atoms—these are molecules as compounds. Water (H2O) is the go-to example: two hydrogen atoms bonded to one oxygen atom. Glucose (C6H12O6), the sugar that fuels our cells, is a molecule with carbon, hydrogen, and oxygen atoms bonded in a very specific arrangement.
Remember: molecules are exactly two or more atoms stuck together and bonded in a way defined by a formula. This formula is not just cosmetic; it reveals the exact numbers of each atom in the molecule.
Why Not All Structures Are Molecules
Before we get carried away, it’s important to clarify something that often trips people up. Not everything with atoms bonded together is a molecule. Some compounds form big, repeating structures rather than discrete molecules.
Ionic compounds like salt (NaCl) don’t form molecules. Instead, they create lattice structures—think of an endless grid of sodium and chloride ions stacked in a repeating pattern. A salt crystal doesn’t contain a “molecule of salt,” it’s just a repeating network.
Covalently bonded networks, like quartz (SiO2), also differ. Here, silicon and oxygen atoms bond in huge 3D networks. The formula tells you the ratio of atoms but not discrete clusters—there are no distinct molecules floating around.
This distinction matters because molecules are identifiable, countable units with fixed numbers of atoms. Lattices and networks extend endlessly in all directions. So, while molecules are the ‘building blocks’ in many contexts, crystals and networks are gigantic structures made of the same atoms bonded repeatedly.
The Practical Side: Why Knowing the Difference Matters
Grasping the difference between atoms and molecules is fundamental in chemistry, biology, and even daily life. When a chemist talks about molecules, they mean something very specific. This precision helps with everything from drug design to understanding the air pollution outside your window.
For example, when you breathe in oxygen, you inhale O2 molecules, not just free atoms. The bonding in molecules affects how substances interact, behave, and transform. If molecules could break apart into single atoms easily, life as we know it would be very different—or downright impossible.
Understanding ionic lattices versus molecular compounds also impacts industries. Salt’s lattice structure gives it high melting points and solubility attributes different from molecular substances like sugar.
Want to See It in Action? Visual Learning Helps
Sometimes, text alone isn’t enough. Watching animated videos can make these tiny concepts click instantly. Check out this resource Atoms and Molecules 101 for a clear, visual explanation that supplements what we covered.
Wrapping it Up: Quick Recap
- Atom: The smallest particle that still defines a chemical element.
- Molecule: Two or more atoms chemically bonded as a distinct entity defined by a chemical formula.
- Types of Molecules: Made of one kind of atom (O2) or multiple kinds (H2O).
- Non-molecular structures: Ionic lattices (NaCl) and covalent networks (SiO2) are not molecules but extended structures.
Next time you look at your glass of water or breathe fresh air, know that countless atoms and molecules buzz invisibly around and within you, forming the essence of life and matter. Understanding their tiny world gives us insight into the big picture. Fascinating, isn’t it?
What makes an atom different from a molecule?
An atom is the smallest part of an element. A molecule is made when two or more atoms bond together in a specific way.
Can a molecule contain atoms of only one type?
Yes, some molecules consist of only one kind of atom, like O₂ (oxygen gas) or S₈ (sulfur ring).
Are all chemical compounds molecules?
No. Some compounds, like ionic ones, form lattice structures, not molecules. For example, NaCl forms a lattice, not discrete molecules.
How do molecules differ from covalent network structures?
Molecules are distinct groups of atoms bonded in fixed numbers. Covalent networks are giant, continuous structures made of atoms bonded throughout.
What defines the exact number of atoms in a molecule?
The chemical formula shows the exact number and types of atoms in a molecule. This ratio doesn’t apply to lattices or networks.



Leave a Comment