What Is a Molecule? Defining the Term
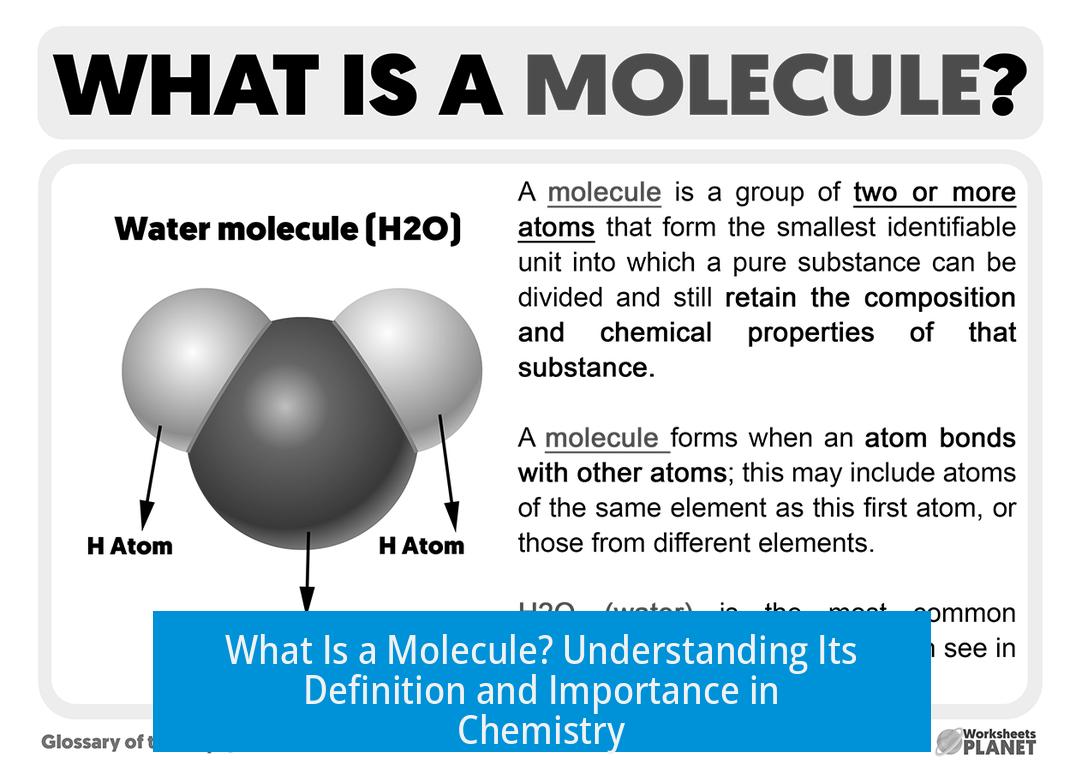
A molecule is a group of two or more atoms held together exclusively by covalent bonds. This definition distinguishes molecules from other chemical groupings such as ionic compounds, complexes, or macromolecules. Covalent bonding binds atoms by sharing electrons, forming discrete entities recognized as molecules.
Common Usage and Definitions
In everyday chemistry, “molecule” typically refers to entities connected by covalent bonds. For example, oxygen (O2) and water (H2O) are molecules because their atoms share electrons. Conversely, ionic compounds like sodium chloride (NaCl) consist of ions held together by electrostatic forces and are called salts, not molecules.
- Groups involving metal ions bonded non-covalently, such as metal complexes, are generally not termed molecules.
- Macromolecules, which contain repeating units and extensive bonding networks, are classified separately from typical molecules.
- Organometallic compounds blur lines, but molecules usually require covalent bonding among all constituent atoms.
Challenges in Defining Molecules via Chemical Bonding
Using chemical bonds or forces to define molecules poses problems. Chemical bonds are conceptual models, not sharply defined physical entities. Theories about bonding interpretations vary and lack universal agreement. This ambiguity means definitions relying on bonds or forces are somewhat fuzzy and open to debate.
Alternative Theoretical Perspectives
One approach is based on Bader’s Atoms in Molecules (AIM) theory. AIM defines atoms in a molecule by regions of space with specific electron density characteristics. This method emphasizes measurable electronic properties rather than unclear bonding concepts, providing a clearer framework to describe molecules objectively.
Practical Naming and Teaching Considerations
While theoretical definitions vary, practical chemistry requires consistency. Covalent arrangements are called molecules, ionic groupings are called salts or complexes. Teaching chemistry often follows conventions from textbooks. Organic chemistry focuses heavily on molecules due to covalent bonding’s prevalence in that field.
Key Takeaways
- A molecule forms from two or more atoms linked by covalent bonds.
- Ionic compounds and metal complexes usually are not molecules by strict definition.
- Chemical bonds and forces are conceptual and lead to fuzzy boundaries in definitions.
- Bader’s Atoms in Molecules offers a physics-based alternative to describe molecules.
- Practical usage aligns with bonding type and field conventions for clarity.
What defines a molecule according to covalent bonding?
A molecule consists of two or more atoms held together exclusively by covalent bonds. This means atoms share electrons to form stable groups.
How do molecules differ from complexes or ionic compounds?
Complexes involve metal and non-covalent bonds. Ionic compounds are made of charged ions and are not called molecules but salts or ionic compounds instead.
Why is the concept of a molecule considered fuzzy in chemistry?
Chemical bonding and forces aren’t defined by strict physical laws alone. Different theories interpret bonds variedly, causing debates about what exactly makes a molecule.
What is Bader’s Atoms in Molecules theory?
This theory defines molecules based on atoms with clear energies. It avoids ambiguous bonding terms by focusing on energy and spatial distribution within atoms.
How do naming conventions vary in chemistry?
Covalent groupings are typically called molecules. Soluble ionic groupings without hydroxides are called salts. Terms depend on the field and teaching consistency.


Leave a Comment