What Is Electronegativity Exactly?
Electronegativity is the ability of an atom to attract electrons within a shared covalent bond. This concept explains how atoms pull electrons toward themselves when bonded, influencing bond polarity and molecular properties. However, electronegativity is not a fixed value; it varies depending on the scale and context used to define it.
Defining Electronegativity
At its core, electronegativity describes how strongly an atom attracts shared electrons in a covalent bond. The stronger the attraction, the more polarized the bond becomes. This polarization affects molecular interactions and shapes chemical behavior.
Despite this general understanding, electronegativity is not a single absolute number. Different definitions and calculation methods exist, leading to various electronegativity scales. Each scale reflects a slightly different aspect of electron attraction.
The Pauling Scale: The Most Common Reference
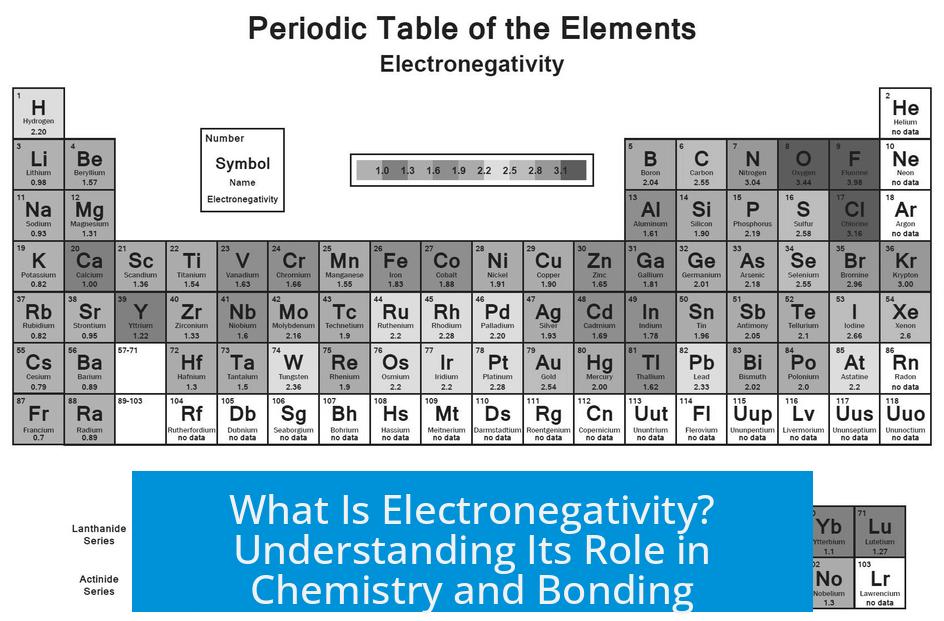
The Pauling scale is the original and most widely used electronegativity scale. It bases electronegativity on bond energies, specifically comparing the energy of a heteronuclear A-B bond to the energies of the corresponding homonuclear A-A and B-B bonds.
- The A-B bond is typically stronger than the average of A-A and B-B bonds.
- This difference in bond energy corresponds to the difference in electronegativity between the two atoms.
Pauling’s method quantifies electronegativity by relating it to the extra stability gained when two different atoms form a bond compared to bonds between identical atoms. High Pauling electronegativity indicates a strong pull on bonded electrons.
Electronegativity as a Concept, Not an Absolute
It is important to recognize electronegativity as a conceptual tool rather than a fixed physical property. Several scales exist beyond Pauling’s, such as Mulliken or Allred-Rochow scales, each based on different parameters like atomic ionization energies or effective nuclear charge.
The variations highlight that electronegativity depends partly on context: the chemical environment, the kind of bond, and even how values are calculated.
Electronegativity’s Role in Chemical Bonding
Electronegativity helps explain the polarization within covalent bonds. When two atoms with different electronegativities bond, electrons spend more time near the more electronegative atom, creating partial charges.
This polarity affects molecular shape, dipole moments, and intermolecular forces. For example, in water (H2O), oxygen’s higher electronegativity compared to hydrogen results in a polar molecule.
Yet, electronegativity alone does not fully describe bonding.
Limitations: Beyond Electronegativity
Several key factors influence bonding strength and character besides electronegativity:
- Atomic Size: Smaller atoms concentrate charge more effectively. Nitrogen, oxygen, and fluorine are good hydrogen bond acceptors partly due to their small radii.
- Orbital Overlap: Bonds require proper orbital shapes and sizes to overlap. For example, chlorine’s 3p orbitals are large and diffuse, resulting in poor bonding overlap with the hydrogen 1s orbital.
- Bond Type: Hydrogen bonds are partly electrostatic but depend strongly on covalent character through orbital interactions. Good orbital overlap enhances bond strength.
These factors explain cases where electronegativity values do not predict bonding behavior fully. Chlorine, despite its electronegativity, forms weaker hydrogen bonds than oxygen or nitrogen due to poor orbital overlap.
Electronegativity in Molecular Interactions
Electronegativity must be considered with molecular context to understand bonding role and strength:
- Chlorine can act as a hydrogen bond acceptor in molecules like chloroacetic acid, which has a higher melting point than acetic acid, showing stronger intermolecular interactions.
- Fluorine, while very electronegative, often forms weak hydrogen bonds when bonded to carbon. For example, compounds like PFAS (perfluoroalkyl substances) display low solubility partly due to poor hydrogen bonding despite fluorine’s electronegativity.
Thus, molecular structure, bonding environment, and orbital properties crucially modify how electronegativity manifests in real compounds.
Summary Table: Electronegativity Key Points
| Aspect | Details |
|---|---|
| Definition | Ability of an atom to attract shared electrons in a covalent bond. |
| Main Scale | Pauling scale, based on bond energy differences. |
| Conceptual Nature | Electronegativity varies by scale and context; not a fixed property. |
| Role in Bonding | Explains electron polarization and bond dipoles. |
| Limitations | Atomic size, orbital overlap, and bonding type also influence bonding strength. |
| Real-World Examples | Chlorine forms weak hydrogen bonds due to poor orbital overlap despite electronegativity. |
Key Takeaways
- Electronegativity quantifies the pull of an atom on electrons within a bond.
- The Pauling scale remains the most common electronegativity measure.
- Electronegativity is a conceptual tool, not a fixed property.
- Bond polarity arises from differences in electronegativity.
- Atomic size and orbital overlap strongly affect bond strength.
- Electronegativity alone cannot fully predict bonding or hydrogen bond strength.
What exactly does electronegativity measure in chemistry?
Electronegativity measures an atom’s ability to attract electrons within a shared covalent bond. It reflects how strongly the atom pulls electron density toward itself when bonded to another atom.
Why are there different electronegativity scales?
Electronegativity is not a fixed number because it depends on how it’s defined or calculated. Various scales, like the Pauling scale, use different methods based on bond energies or atomic properties.
How does the Pauling scale define electronegativity?
The Pauling scale calculates electronegativity from bond energies. It compares the energy of a bond between two different atoms to the average energy of bonds between the same atoms, showing that mixed bonds are stronger.
Can electronegativity alone predict hydrogen bonding strength?
No. While higher electronegativity helps, hydrogen bonding also depends on atomic size and orbital overlap. Good orbital matching is essential for strong hydrogen bonds.
Why is electronegativity called a conceptual idea rather than an exact value?
Electronegativity varies with the method used to measure it and the context of the bond. Different scales offer different values, making it more of a useful concept than a fixed physical property.



Leave a Comment