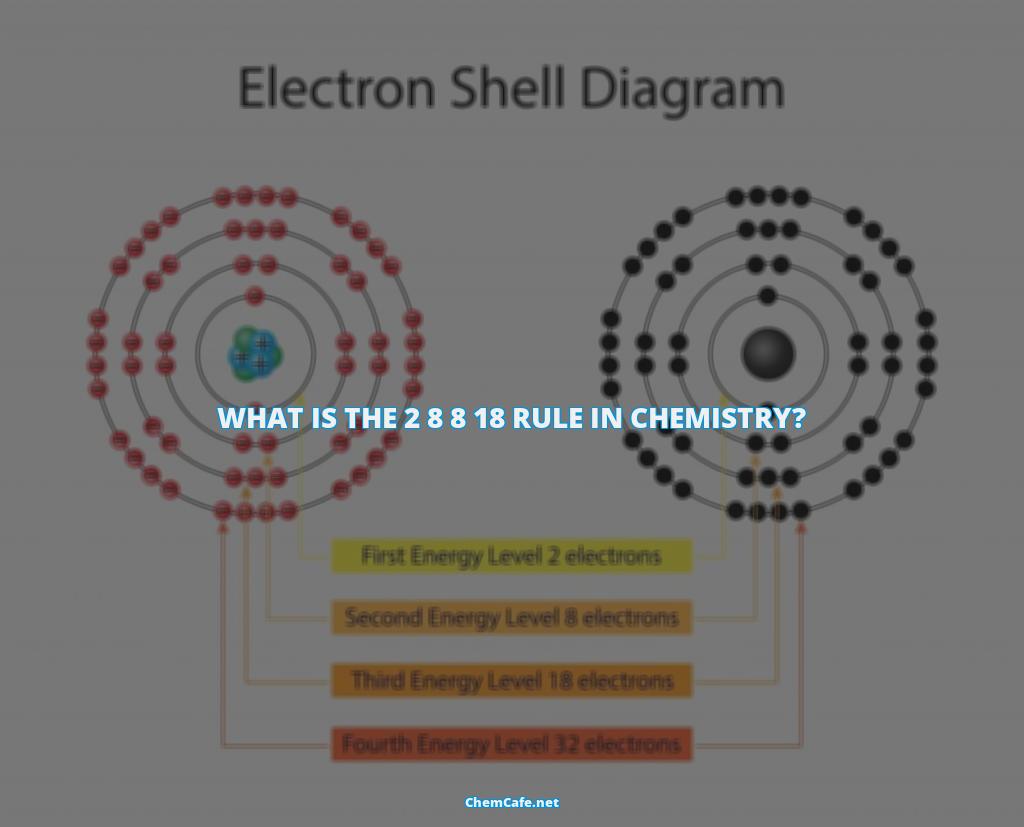
The 2 8 8 18 rule in chemistry is one of the fundamental principles of understanding chemical reactions. It states that the maximum number of electrons that can occupy the outermost shell of an atom is eight. This rule is often referred to as the octet rule, and is a major concept in chemical bonding.
Atoms are composed of protons and neutrons in the nucleus, and electrons in shells surrounding the nucleus. The electrons in the outermost shell determine the chemical properties of the atom. The 2 8 8 18 rule in chemistry states that the outermost shell of an atom can contain a maximum of eight electrons. When an atom has less than eight electrons in its outermost shell, it is said to be ‘unsaturated’ and will tend to react in order to achieve a full outer shell, with eight electrons.
The 2 8 8 18 rule in chemistry is particularly important in understanding the behavior of atoms in the first 18 elements of the periodic table. For the first 18 elements, there is a 2-8-8 rule; the first shell can contain up to two electrons, the second shell can contain up to eight, and the third shell can contain up to eight electrons. You can see that sodium (Na) and magnesium (Mg) have a couple of extra electrons.
The 2 8 8 18 rule in chemistry can help us understand why elements react in certain ways. For example, according to the octet rule, elements tend to react in order to obtain a full outer shell, whether by sharing, losing or gaining electrons. The 1st, 2nd, and 3rd shells can each hold up to 2, 8 and 8 electrons, respectively. Once a shell is filled up, any further electrons must fill in a new higher level shell.
The 2 8 8 18 rule in chemistry is an important concept to understand when it comes to chemical reactions. It can help us understand why atoms react in certain ways, and why some elements have different configurations from others. This rule is also important to consider when it comes to understanding the properties of different elements, and how they interact with each other.
What is the 2 8 8 18 rule in chemistry?
The 2 8 8 18 rule in chemistry is a rule that describes the maximum number of electrons that can be present in each shell of an atom. This rule states that the first shell can hold up to two electrons, the second shell can hold up to eight electrons, the third shell can hold up to eighteen electrons, and so on. This rule is also referred to as the Octet Rule, due to the fact that the outermost shell of each atom typically contains eight electrons.
What is the 2 8 8 electron rule?
The 2 8 8 electron rule states that the first shell of an atom can contain up to two electrons, the second shell can contain up to eight electrons, and the third shell can contain up to eight electrons. This rule applies to atoms that have atomic numbers between 1 and 18. Examples of such atoms include sodium (Na) and magnesium (Mg).
Why is the electron configuration 2 8 8 18?
The electron configuration 2 8 8 18 describes the arrangement of electrons in various shells, sub-shells, and orbitals in an atom. This arrangement is written as 2, 8, 8, 18, 18, 32, and it is written as nlx (where n indicates the principal quantum number, l indicates the azimuthal quantum number or sub-shell, and x is the number of electrons).
What is the 2 8 8 Rule Definition?
The 2 8 8 Rule Definition states that atoms that have atomic numbers between 1 and 18 obey a 2-8-8 rule. This means that the first shell is filled with two electrons, the second shell is filled with eight electrons, and the third shell is filled with eight electrons. Examples of such atoms include sodium (Na) and magnesium (Mg).
What is the 2 8 8 2 rule in chemistry?
The 2 8 8 2 rule in chemistry states that elements will react in ways to obtain a full outer shell. This can be done by sharing, losing, or gaining electrons. The first, second, and third shells can hold up to two, eight, and eight electrons respectively. Once a shell is filled with electrons, any additional electrons must be placed in a new higher-level shell.
What is the 2 8 8 18 rule in chemistry?
The 2 8 8 18 rule in chemistry is also known as the Octet Rule. This rule states that atoms prefer to have eight electrons in their valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. The Octet Rule applies to the main group elements, as they have electron configurations that end with (s^2p^6).
What is the 2 8 8 2 rule in chemistry?
The 2 8 8 2 rule in chemistry states that elements will react in ways to obtain a full outer shell. This can be done by sharing, losing, or gaining electrons. The first, second, and third shells can hold up to two, eight, and eight electrons respectively. Once a shell is filled with electrons, any additional electrons must be placed in a new higher-level shell.
Why do the electron shells fill to 2 8 8 8 instead of filling their shells completely?
The electron shells fill to 2 8 8 8 instead of filling their shells completely since this arrangement is most stable. Atoms prefer to have a full octet (eight electrons) in their outermost shell. Therefore, when an atom has fewer than eight electrons in its outermost shell, it will react in order to obtain a full octet. This reaction can involve sharing, losing, or gaining electrons.
What is 2,8,8,18,18,32,32,50…..?????????
The sequence 2, 8, 8, 18, 18, 32, 32, 50….. is the electron configuration of an atom. This sequence indicates the number of electrons present in each shell of the atom. The first number (2) represents the number of electrons in the first shell, the second number (8) represents the number of electrons in the second shell, and so on.
Why is 3rd shell 8 or 18?
The third shell of an atom is 8 or 18 because atoms in the second and third periods can accommodate eight and eighteen electrons respectively. Since the outermost shells of these atoms can contain only eight electrons, there are only eight elements in both periods.
Why potassium has electron configuration 2.8 8.1 and not 2.8 9?
The electron configuration of potassium is 2.8 8.1 and not 2.8 9 because of the octet rule. According to the octet rule, the outermost shell of an atom can contain up to eight electrons (except for the K shell, which can contain up to two electrons). Therefore, the electronic configuration of potassium is 2, 8, 8, 1 and not 2, 8, 9.
What is the 2 8 8 rule in chemistry?
Chemistry is a fascinating subject that can be studied in various ways, from the chemical reactions of elements to the different structures of molecules. One of the core principles of chemistry is the 2 8 8 rule, which is used to explain the arrangement of electrons in an atom’s outer shell. This rule has been around since the early 1900s and is still an important part of the discipline today.
What is the 2 8 8 Rule Definition?
The 2 8 8 rule is a scientific principle that states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eight electrons. This rule is sometimes referred to as the “octet rule” since it states that the number of electrons in the outermost shell of an atom is eight.
The 2 8 8 rule is important in chemistry because it provides a way to predict the behavior of atoms during chemical reactions. The rule states that atoms tend to form chemical bonds in order to achieve a full outer shell, which is more stable than having an incomplete outer shell. This means that atoms will either share, lose, or gain electrons in order to achieve a full outer shell.
What is the 2 8 8 electron rule?
The 2 8 8 electron rule is a scientific principle that states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eight electrons. This rule is sometimes referred to as the “octet rule” since it states that the number of electrons in the outermost shell of an atom is eight.
The 2 8 8 electron rule is important in chemistry because it provides a way to predict the behavior of atoms during chemical reactions. The rule states that atoms tend to form chemical bonds in order to achieve a full outer shell, which is more stable than having an incomplete outer shell. This means that atoms will either share, lose, or gain electrons in order to achieve a full outer shell.
What is the 2 8 8 2 rule in chemistry?
The 2 8 8 2 rule in chemistry is a scientific principle that states that the outermost shell of an atom can contain a maximum of two electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains two electrons. This rule is sometimes referred to as the “octet rule” since it states that the number of electrons in the outermost shell of an atom is eight.
The 2 8 8 2 rule is important in chemistry because it provides a way to predict the behavior of atoms during chemical reactions. The rule states that atoms tend to form chemical bonds in order to achieve a full outer shell, which is more stable than having an incomplete outer shell. This means that atoms will either share, lose, or gain electrons in order to achieve a full outer shell.
What is the 2 8 8 18 rule in chemistry?
The 2 8 8 18 rule in chemistry is a scientific principle that states that the outermost shell of an atom can contain a maximum of eighteen electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eighteen electrons. This rule is sometimes referred to as the “octet rule” since it states that the number of electrons in the outermost shell of an atom is eight.
The 2 8 8 18 rule is important in chemistry because it provides a way to predict the behavior of atoms during chemical reactions. The rule states that atoms tend to form chemical bonds in order to achieve a full outer shell, which is more stable than having an incomplete outer shell. This means that atoms will either share, lose, or gain electrons in order to achieve a full outer shell.
Why do the electron shells fill to 2 8 8 8 instead of filling their shells completely?
The reason that the electron shells fill to 2 8 8 8 instead of filling their shells completely is because of the octet rule. The octet rule states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eight electrons. This rule is sometimes referred to as the “octet rule” since it states that the number of electrons in the outermost shell of an atom is eight.
When atoms have fewer than eight electrons in their outermost shell, they will react and form more stable compounds. This is because atoms with a full outer shell are more stable than those with an incomplete outer shell. Therefore, when atoms have fewer than eight electrons in their outermost shell, they will react and form more stable compounds by either sharing, losing, or gaining electrons.
Which of the following elements have 2 8 8 electronic configuration?
The elements that have a 2 8 8 electronic configuration are Potassium (K), Calcium (Ca), Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), and Zinc (Zn).
What is the most important rule in chemistry?
The most important rule in chemistry is the law of conservation of mass. This law states that matter can neither be created nor destroyed, but can only be changed in form. This law is important in chemistry because it serves as the foundation for understanding how atoms interact with each other and how chemical reactions take place.
Why is calcium 2 8 8 2?
Calcium is 2 8 8 2 because it follows the octet rule. The octet rule states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains two electrons. This rule is sometimes referred to as the “octet rule” since it states that the number of electrons in the outermost shell of an atom is eight.
What is 2 8 8 in periodic table?
2 8 8 in the periodic table refers to the electronic configuration of an element. The electronic configuration of an element is the arrangement of electrons in an atom’s outer shell. The 2 8 8 configuration refers to the first shell containing two electrons, the second shell containing eight electrons, and the third shell containing eight electrons. This rule is sometimes referred to as the “octet rule” since it states that the number of electrons in the outermost shell of an atom is eight.
What is 2,8,8,18,18,32,32,50…..?????????
2,8,8,18,18,32,32,50….. is a pattern of electron configurations for the elements in the periodic table. This pattern follows the octet rule, which states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eight electrons. As you move across the periodic table, the number of electrons in the outermost shell increases: 2, 8, 8, 18, 18, 32, 32, 50…..
What comes after 2 8 8?
The pattern 2 8 8 is followed by 18, 18, 32, 32, 50 in the periodic table. This pattern follows the octet rule, which states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eight electrons. As you move across the periodic table, the number of electrons in the outermost shell increases: 2, 8, 8, 18, 18, 32, 32, 50…..
Which element is 2 8 8 8?
The element that is 2 8 8 8 is Argon (Ar). Argon has an electronic configuration of 2 8 8 8, which follows the octet rule. The octet rule states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eight electrons.
Why is it 2 8 8 1 and not 2 8 9?
The reason it is 2 8 8 1 and not 2 8 9 is because of the octet rule. The octet rule states that the outermost shell of an atom can contain a maximum of eight electrons. This means that the first shell of an atom contains two electrons, the second shell contains eight electrons, and the third shell contains eight electrons. Therefore, the electronic configuration of potassium is 2 8 8 1 and not 2 8 9.
What is the magic rule in chemistry?
The magic rule in chemistry is the law of conservation of mass. This law states that matter can neither be created nor destroyed, but can only be changed in form. This law is important in chemistry because it serves as the foundation for understanding how atoms interact with each other and how chemical reactions take place.
In conclusion, the 2 8 8 rule is an important scientific principle in chemistry that explains the arrangement of electrons in the outermost shell of an atom. This rule states that atoms tend to form chemical bonds in order to achieve a full outer shell, which is more stable than having an incomplete outer shell. This means that atoms will either share, lose, or gain electrons in order to achieve a full outer shell. The 2 8 8 rule is important in chemistry because it provides a way to predict the behavior of atoms during chemical reactions.
What is the rule of 8 in atoms?
Atoms are the building blocks of all matter. They are the smallest particles in the universe, and they contain the elements that make up everything from the stars in the sky to the rocks on the ground. The rule of 8 in atoms states that atoms are at their most stable when they have eight electrons in their outer shell, giving them the electron configuration of a noble gas.
What is the Octet Rule?
The octet rule is a rule of thumb in chemistry that states that atoms are more stable when they have eight valence electrons in their outer shell. This is because the eight electrons fill out the orbitals in the valence shell, and the atom is then in the same electron configuration as the noble gases. The noble gases are the group of elements on the right side of the periodic table and are the most stable elements due to their full outer shell of electrons.
How Does the Octet Rule Apply to Drawing Lewis Structures?
We can use the octet rule to help us draw Lewis diagrams. When assigning electrons to atoms, make sure that each atom has eight electrons in its outer shell. This is usually accomplished by making sure that each atom has the same number of electrons as its group number on the periodic table. For example, oxygen is in group 6, so it should have six electrons in its outer shell.
Exceptions to the Octet Rule
There are some exceptions to the octet rule. Molecules with odd numbers of electrons disobey the octet rule. These include free radicals such as nitric oxide and nitrogen dioxide. Free radicals are molecules with an unpaired electron, and they are highly reactive.
Some atoms can form incomplete octets with fewer than eight valence electrons. These include boron and aluminium. Hydrogen, helium and lithium always form incomplete octets with just two outer shell electrons.
Some atoms can form expanded octets with more than eight valence electrons. The extra electrons go in a d-subshell. Molecules with expanded octets include phosphorus pentachloride and xenon tetrafluoride.
Conclusion
The octet rule is an important rule in chemistry, as it explains why atoms form molecules and compounds. The octet rule states that atoms are more stable when they have eight electrons in their outer shell. This is usually accomplished by making sure that each atom has the same number of electrons as its group number on the periodic table. There are some exceptions to the octet rule, such as free radicals and molecules with expanded octets. Understanding the octet rule is essential for understanding the structure of molecules.
What is the 2n 2 rule in chemistry?
The 2n2 rule in chemistry is a rule of thumb used to predict the number of electrons that an atom can hold in the outermost shell of its electron configuration. It states that an atom can hold up to a maximum of 2n2 electrons, where “n” is the number of the outermost shell. This means that for the first shell, “n” is equal to one and the maximum number of electrons it can hold is 2(1)2 or four electrons. For the second shell, “n” is equal to two and the maximum number of electrons it can hold is 2(2)2 or eight electrons. This rule is also known as the octet rule or the octet-rule of valence shell electron configuration.
Understanding the 2n2 Rule
The 2n2 rule is based on the fact that atoms tend to bond in order to achieve a stable, octet configuration. This is because atoms tend to gain, lose, or share electrons in order to reach a state of having eight electrons in their outermost shell, which is considered to be the most stable arrangement. This is why the 2n2 rule is also known as the octet rule.
Atoms can gain, lose, or share electrons in order to reach a stable, octet configuration. For example, an atom with five electrons in its outermost shell, such as nitrogen, can gain three electrons to achieve a full, octet configuration. On the other hand, an atom with seven electrons in its outermost shell, such as fluorine, can lose one electron to achieve a full, octet configuration.
How the 2n2 Rule is Used in Chemistry
The 2n2 rule is used in chemistry to predict the number of bonds that an atom can form. Knowing the number of electrons in an atom’s outermost shell allows chemists to predict the number of bonds that the atom can form with other atoms. For example, an atom with four electrons in its outermost shell, such as carbon, can form up to four covalent bonds. This is because each covalent bond consists of two electrons, so four covalent bonds would require a total of eight electrons.
The 2n2 rule is also used to predict the type of bond that a given atom can form. For example, an atom with six electrons in its outermost shell, such as oxygen, can form two covalent bonds and two single bonds. This is because two of the electrons will be shared between the two atoms to form the covalent bond, while the other two electrons will be used to form the single bonds.
Exceptions to the 2n2 Rule
The 2n2 rule is generally applicable to most atoms, but there are exceptions. For example, some atoms can exceed the maximum number of electrons in their outermost shell. This occurs when an atom is in an excited state, meaning that it has absorbed energy which has caused it to move to a higher energy level. Since the outermost shell is not filled, the atom can hold more than the maximum number of electrons.
The 2n2 rule is also not applicable to transition metals, which are atoms that are located in the middle of the periodic table. These atoms have partially filled d-orbitals, which means that they can form more than eight bonds. Therefore, the 2n2 rule does not apply to transition metals.
Conclusion
The 2n2 rule is an important rule in chemistry that is used to predict the number of electrons an atom can hold in its outermost shell. It states that an atom can hold up to a maximum of 2n2 electrons, where “n” is the number of the outermost shell. The 2n2 rule is used to predict the number of bonds that an atom can form and the type of bond it can form. However, there are exceptions to the 2n2 rule, such as atoms in an excited state and transition metals.
What is 8 electron rule?
The 8 electron rule, also known as the octet rule, is a fundamental principle of chemistry that dictates the most stable electron configuration of atoms. This rule states that atoms will react to become more stable by forming bonds until they have eight electrons in their outermost shell. The 8 electron rule is based on the fact that the most stable arrangement of electrons around an atom is to have eight electrons in the outermost shell.
What is the octet rule in simple words?
The octet rule can be simply stated as: atoms will be most stable when their valence shells are filled with eight electrons. This is because an atom with eight electrons in its outermost shell is similar to the electron configuration of a noble gas, which is a very stable state.
What elements break the octet rule?
Atoms such as hydrogen, beryllium, and boron do not have enough electrons to achieve an octet and are therefore exceptions to the octet rule. Hydrogen only has one valence electron and can only form one bond. Beryllium has two valence electrons and can only form two bonds. Boron has three valence electrons but can only form three bonds.
The Octet Rule – Chemistry LibreTexts
The octet rule was first proposed by Richard Abegg in 1904, who stated that the difference between the maximum positive and negative valences of an element is often eight. This statement was later expanded upon by Gilbert N. Lewis in 1916 when he proposed the cubical atom theory, which suggested that atoms will react to become more stable by forming bonds until they have eight electrons in their outermost shell.
Why do atoms need 8 electrons to be stable?
Atoms with eight electrons in their valence shell have completely filled last orbitals and are therefore the most stable, as their electronic configuration is similar to that of the closest noble gas. Atoms with fewer than eight electrons in their valence shell are less stable because the shells are not completely filled.
What is the octet rule in chemistry?
The octet rule in chemistry states that atoms are most stable when their valence shells are filled with eight electrons. This rule applies to compounds containing carbon, nitrogen, oxygen, and fluorine. When one of these atoms has less than eight valence electrons it has an open octet.
How many shells do 8 electrons need?
The first shell (closest to the nucleus) can hold two electrons. The second shell can hold eight electrons. The third shell can hold 32 electrons.
How do you satisfy the octet rule?
Atoms satisfy the octet rule by either sharing their valence electrons with other atoms or transferring valence electrons from one atom to another. Atoms with fewer than eight electrons in their valence shell will tend to form bonds with other atoms to achieve the octet rule.
What happens when you have 8 valence electrons?
When an atom has eight valence electrons it is said to have a complete octet. A complete octet is very stable because all orbitals will be full. Atoms with greater stability have less energy, so a reaction that increases the stability of the atoms will release energy in the form of heat or light.
What are the 4 exceptions to the octet rule?
The four exceptions to the octet rule are: hydrogen, beryllium, boron, and odd-electron molecules. Hydrogen, beryllium, and boron have too few electrons to form an octet. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Examples of stable odd-electron molecules are NO, NO2, and ClO2.
Who violates the octet rule?
Atoms with fewer than eight electrons in their valence shell will tend to form bonds with other atoms to achieve the octet rule. This means that atoms with fewer than eight electrons are the ones that violate the octet rule. Examples of atoms that violate the octet rule are hydrogen, beryllium, and boron.
What is octet rule and why is it important?
The octet rule is an important principle that states that atoms which have bonded share eight outer electrons. This means that the atom’s valence shell has a resemblance with a noble gas. This rule is important because it helps us predict the most stable electron configuration of atoms and therefore helps us understand how atoms will react with each other.
The 8 electron rule is an important principle of chemistry that helps us predict the most stable electron configuration of atoms. Atoms that have fewer than eight electrons in their outermost shell will react to become more stable by forming bonds until they have eight electrons in their outermost shell. This rule applies to compounds containing carbon, nitrogen, oxygen, and fluorine and is the basis of understanding how atoms interact with each other. Knowing the 8 electron rule is essential for understanding and predicting the behavior of atoms.
Why are there 8 electrons in a shell?
One of the most fundamental rules in chemistry is the octet rule, which states that atoms tend to bond in such a way that their outermost electron shell is full, or “octet”. This rule is based on the observation that most elements important in biology need eight electrons in their outermost shell in order to be stable. But why do the electron shells fill to 2 8 8 8 instead of filling their shells completely?
The Electron Configuration
Atoms are composed of protons, neutrons, and electrons. The protons and neutrons are located in the nucleus of the atom, and the electrons are arranged in shells around the nucleus. The electrons in the outermost shell, known as the valence shell, can easily interact with other atoms to form chemical bonds. The order in which the electrons are filled in the shells is known as the electron configuration.
The Octet Rule
The octet rule states that chemical elements tend to bond in such a way that their outermost electron shell is full. This means that atoms will try to fill their valence shells with eight electrons. The octet rule is only applicable to the main group elements, which have atomic numbers of 1 through 18. These elements have a maximum of eight electrons in their valence shell and will try to fill it to capacity.
Why Eight Electrons?
So why can the outermost shell have no more than eight electrons? The answer lies in the electron properties and the nucleus properties. When we observe the periodic table, we find that d and f electrons are added in (n−1) and (n−2) shells, and only s and p electrons are added in the nth, or outermost shell. This means that an element can have no more than eight electrons in its valence shell.
The 2 8 8 Rule
The 2 8 8 rule states that the first two periods of the periodic table can accommodate 8 and 18 electrons respectively. This means that the first two shells can accommodate a maximum of 8 and 18 electrons, and the third shell can accommodate a maximum of 8 electrons. When the other shells get filled and the resultant number of electrons becomes 18, it gets added up and settles in the third electron shell, and three shells are acquired by the fourth period.
The Importance of Eight Electrons
So why is it important for atoms to have eight electrons in their outermost shell? Since all noble gases have eight electrons in their valence shell, they are observed to be highly stable and inert, i.e., they do not react with other elements. Thus, the octet rule states that elements will try to fill their valence shells with eight electrons in order to be stable. Elements with less than eight electrons in the valence shell are less stable.
Atoms With More Than Eight Electrons
It is possible for atoms to have more than eight electrons in the outermost shell, but it is not common. This is because elements with atomic numbers greater than 18 can fill their outermost shells with more than eight electrons. For example, sodium (Na) and magnesium (Mg) have a couple of extra electrons in their outermost shells.
Conclusion
In conclusion, the octet rule states that atoms tend to bond in such a way that their outermost electron shell is full, or “octet”. This rule is based on the observation that most elements important in biology need eight electrons in their outermost shell in order to be stable. The octet rule is only applicable to the main group elements, which have atomic numbers of 1 through 18, and which can have a maximum of eight electrons in their valence shell. Elements with less than eight electrons in the valence shell are less stable, so atoms will try to fill their valence shells with eight electrons in order to be stable. It is possible for atoms to have more than eight electrons in the outermost shell, but it is not common.
What is the magic rule in chemistry?
Chemistry is a complex science, and the periodic table of elements is a key tool for understanding chemical reactions. One of the most important rules of chemistry is the magic number rule, which explains the stability of certain elements and helps scientists better understand the behavior of atoms.
What is the Magic Number Rule?
The magic number rule is a fundamental law of physics that explains the stability of certain numbers of electrons in an atom. According to this rule, certain numbers of electrons are more likely to have a stable configuration than others. These numbers, called magic numbers, are 2, 8, 20, 28, 50, 82, and 126.
Why are these numbers magic?
Atoms with these magic numbers of electrons are especially stable, meaning they are less likely to react with other atoms. This is because electrons within a shell have similar energies and distance from the nucleus, which makes them less likely to form bonds with other atoms.
For example, atoms with 18 electrons in closed-shell configurations, such as the chloride ion (Cl−), the argon atom (Ar), and the potassium ion (K+), are particularly stable and don’t easily react with other atoms. Similarly, the number of electrons present in the neutral atoms of the noble gases correspond to the atomic magic numbers.
The same is true for nuclei, which also have magic numbers. These are 2, 8, 20, 28, 50, 82, and 126, and nuclei with these numbers of protons are especially stable. For example, tin (atomic number 50) has 10 stable isotopes, whereas indium (atomic number 49) and antimony (atomic number 51) have only 2 stable isotopes apiece.
The Octet Rule
The magic number rule is closely related to the octet rule, which states that atoms with eight valence electrons are especially stable and common. This is because of the electron configuration of atoms, which is determined by the number of electrons in the outermost shell.
Atoms with eight valence electrons have a full outermost shell, which is especially stable. This is why many atoms, such as oxygen, carbon, and nitrogen, have eight valence electrons and form bonds with other atoms in order to obtain a full outermost shell.
Magic Numbers for Nuclei
The magic number rule also applies to nuclei, which also have certain numbers of protons and neutrons that generate stable (non-radioactive) isotopes. These are called magic numbers, and they are 2, 8, 20, 28, 50, 82, 114 for protons and 2, 8, 20, 28, 50, 82, 126, 184 for neutrons.
In some cases, there are double magic numbers, which occur when both the protons and neutrons have a magic number. These double magic numbers only occur for heavier isotopes, because the repulsion of the forces between the protons increases with the number of protons.
Exceptions to the Magic Number Rule
While the magic number rule is a useful tool for understanding the stability of certain elements, it is important to note that there are exceptions. Many isotopes with no magic numbers of nucleons are still stable, and there are also violations to the octet rule.
Conclusion
The magic number rule is an important law of physics that explains the stability of certain elements and helps scientists better understand the behavior of atoms. This rule explains why certain numbers of electrons and protons are more stable than others, and why certain elements are more common than others. While there are exceptions to this rule, it is a useful tool for understanding the behavior of atoms.
What is 18 and 16 electron rule?
The 18 and 16 Electron Rule are two postulates or rules for organometallic complexes and their reactions. This rule is used to predict and explain the stability of certain organometallic compounds, which have a central transition metal atom surrounded by multiple organic ligands. This article will discuss the 18 and 16 electron rule in detail, exploring how it can be used to describe the stability of organometallic complexes.
What is the 18 Electron Rule?
The 18 electron rule is primarily used to formulate stable metal complexes, especially organometallic compounds. This rule states that a transition metal organometallic compound will only form when the sum of the metal’s d electrons and the electrons often thought of as coming from the surrounding ligands equals 18. An electron-rich metal and potent pi-acceptors ligands are generally the conditions encouraging adherence to the 18 electron rule.
Explaining the 18 Electron Rule
To explain the 18 electron rule, it is important to understand the electronic configuration of the transition metal. The transition metal atom has a valence shell that can hold up to 18 electrons, which can be divided into s, p, and d orbitals. The d orbitals are the most important, as they are responsible for the stability of the organometallic complex. The 18 electron rule states that in order for the organometallic complex to be stable, the number of electrons in the d orbitals must equal 18.
What is the 16 Electron Rule?
The 16 electron rule applies to organometallic compounds with two or more metal atoms. This rule states that the total number of electrons in the d orbitals of all the metal atoms must equal 16. This is because the d orbitals of each metal atom can only hold up to 8 electrons. Therefore, when two or more metal atoms are present, the total number of electrons in the d orbitals must be 16 in order for the complex to be stable.
Explaining the 16 Electron Rule
The 16 electron rule can be explained using the same principles as the 18 electron rule. However, in this case, the d orbitals of the metal atoms can only hold up to 8 electrons each. Therefore, when two or more metal atoms are present, the total number of electrons in the d orbitals must be 16 in order for the complex to be stable.
Difference between 18 and 16 Electron Rule
The main difference between the 18 and 16 electron rules is the number of electrons in the d orbitals. The 18 electron rule states that the number of electrons in the d orbitals must equal 18, while the 16 electron rule states that the total number of electrons in the d orbitals of all the metal atoms must equal 16.
Examples of 18 and 16 Electron Rule
One example of the 18 electron rule is the formation of a tungsten carbonyl complex. In this example, the tungsten atom has 6 electrons in its d orbitals, which are added to the 12 electrons from the ligands, giving a total of 18 electrons. This indicates that the complex is stable and able to form.
An example of the 16 electron rule is the formation of a molybdenum carbonyl complex. In this example, each molybdenum atom has 8 electrons in its d orbitals, giving a total of 16 electrons. This indicates that the complex is stable and able to form.
Conclusion
The 18 and 16 electron rules are two postulates or rules for organometallic complexes and their reactions. The 18 electron rule states that a transition metal organometallic compound will only form when the sum of the metal’s d electrons and the electrons often thought of as coming from the surrounding ligands equals 18. The 16 electron rule applies to organometallic compounds with two or more metal atoms and states that the total number of electrons in the d orbitals of all the metal atoms must equal 16. Examples of the 18 and 16 electron rule were provided to illustrate how these rules can be used to describe the stability of organometallic complexes.



Leave a Comment