The 8 valence electron rule, also known as the octet rule, is a fundamental concept in chemistry that explains the behavior of atoms and their tendency to form stable compounds. It states that atoms prefer to have eight electrons in their valence shell, and will react and form more stable compounds when they have fewer than eight electrons. This rule is based on the observation that the atoms of the main group elements, such as oxygen, carbon, and nitrogen, will form stable molecules by participating in chemical bonding in such a way that each atom has eight electrons in its valence shell.
Understanding the 8 valence electron rule is essential for anyone studying chemistry, as it can help to explain and predict the behavior of atoms and molecules. The rule was first proposed by German chemist Richard Abegg in 1904 in what is now known as Abegg’s rule, which states that the difference between the maximum positive and negative valences of an element is frequently eight. This rule is particularly useful for the main group elements, as an octet in these atoms corresponds to an electron configuration ending with s2p6.
The 8 valence electron rule is based on the fact that atoms want to become stable by achieving a noble gas configuration. This means that they want to have eight electrons in their valence shell, either by sharing electrons with other atoms or by giving up or gaining electrons. When atoms have fewer than eight electrons, they are considered to be less stable and will react and form more stable compounds. The octet rule does not consider d or f electrons, only the s and p electrons.
The 8 valence electron rule is an important concept that can help to explain and predict the behavior of atoms and molecules. It is a useful tool for anyone studying chemistry, and can be used to understand the behavior of different chemical compounds.
What is the 8 valence electron rule?
The 8 valence electron rule is a rule of thumb in chemistry that describes the behavior of atoms when they form chemical bonds with other atoms. It states that atoms tend to form bonds in such a way that each atom involved in the bond has eight electrons in its valence shell. This rule is also known as the octet rule.
The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its valence shell. This is because atoms with eight electrons in their valence shell are more stable than those with fewer or more electrons.
What is the Octet Rule?
The octet rule is a rule of thumb in chemistry that states that atoms tend to form bonds in such a way that each atom involved in the bond has eight electrons in its valence shell. This rule is based on the observation that the atoms of the main group elements have a tendency to form compounds in which each atom has eight electrons in its valence shell. Atoms with eight electrons in their valence shells are more stable than those with fewer or more electrons.
The 8 valence electron rule applies to atoms of the main group elements, which are elements in groups 1-8 of the periodic table. It does not apply to atoms of the transition metals, inner transition metals, lanthanides and actinides.
Examples
Let’s look at an example of the 8 valence electron rule. Consider the formation of a water molecule, H2O. Each oxygen atom has six electrons in its valence shell. In order to satisfy the octet rule, each oxygen atom must gain two electrons, one from each hydrogen atom. The hydrogen atoms each have one electron in their valence shell and must each lose an electron in order to achieve an octet. After the formation of the bond, each oxygen atom has eight electrons in its valence shell and each hydrogen atom has zero electrons in its valence shell.
Exceptions
While the 8 valence electron rule is a useful rule of thumb, there are exceptions. Atoms of the transition metals and inner transition metals tend to violate the octet rule, forming compounds in which not all atoms have eight electrons in their valence shell. In addition, some atoms may gain or lose more or fewer electrons than necessary to achieve an octet in order to achieve a lower energy state.
FAQs
Q: What is the 8 valence electron rule?
A: The 8 valence electron rule is a rule of thumb in chemistry that states that atoms tend to form bonds in such a way that each atom involved in the bond has eight electrons in its valence shell.
Q: Does the 8 valence electron rule apply to all elements?
A: No, the 8 valence electron rule only applies to atoms of the main group elements, which are elements in groups 1-8 of the periodic table. It does not apply to atoms of the transition metals, inner transition metals, lanthanides and actinides.
What does 8 electrons mean?
The number 8 has an important role in chemistry, and understanding its significance can help us understand the behavior of different elements and compounds. The so-called “2-8-8” rule states that the outermost shell of an atom can contain a maximum of 8 electrons. This rule applies to the first 18 elements in the periodic table, from hydrogen to argon.
The Significance Of “8” In Chemistry
In chemistry, 8 isn’t a lucky number, per se, but, a number that indicates stability. The rule of 8 or the Octet rule is the tendency of atoms to have eight electrons in their valence shell. This means that the outermost shell of an atom must contain 8 electrons if it wants to be stable.
Eight electrons in this final shell allow atoms to be stable and non-reactive. Noble gases, for example, are some of the most non-reactive chemical elements one can find in nature. This is because their outermost shells contain 8 electrons, which gives them a complete outer shell and, therefore, makes them stable.
Can All Energy Levels Hold 8 Electrons?
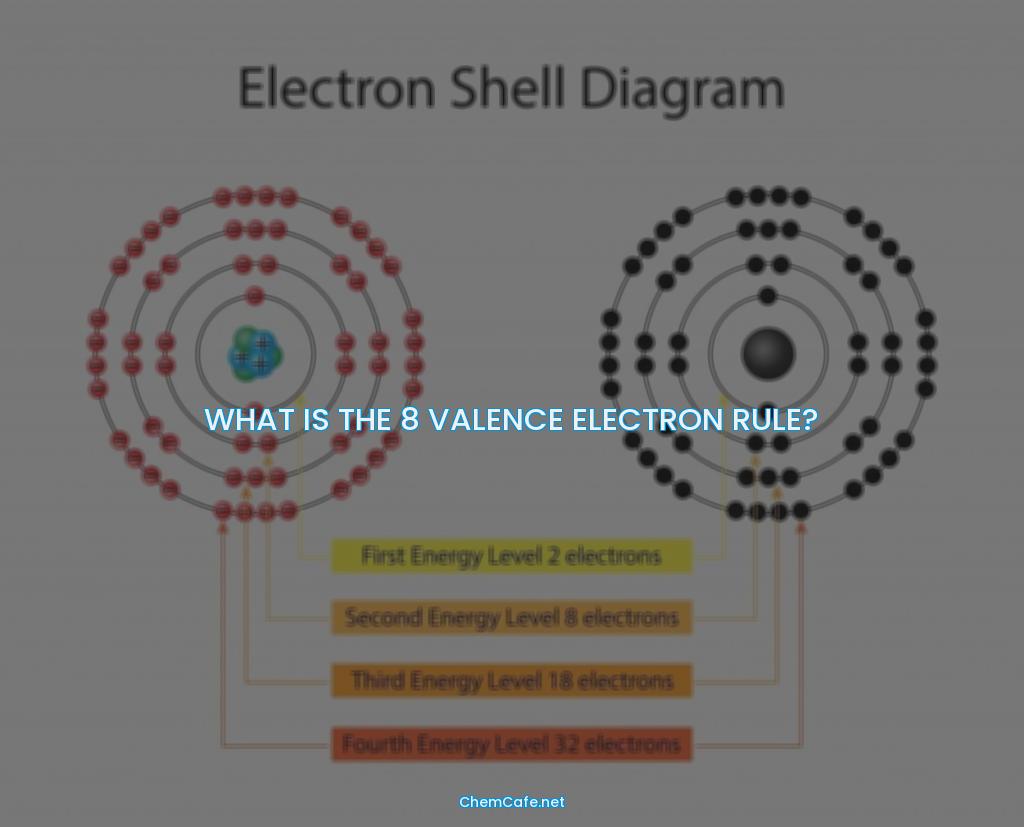
The answer is no. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. The first shell (closest to the nucleus) can hold two electrons. The second shell can hold 8 electrons. The third shell can hold 32 electrons.
But, if you look at the periodic table, it’s easy to see that some elements have more than 8 electrons in their outermost energy level. You can see that sodium (Na) and magnesium (Mg) have a couple of extra electrons. This is because the outermost shell of those atoms is actually the third shell, which can hold up to 32 electrons.
Can 2 Atoms Share 8 Electrons?
Yes, two atoms can share 8 electrons. This is called covalent bonding and it’s one of the most common types of chemical bonds found in nature. In covalent bonding, atoms share electrons in order to achieve a stable configuration.
For example, when two hydrogen atoms form a covalent bond, they share their two electrons in order to form a single bond. Each atom now has a full outer shell of 8 electrons, making both of them stable.
The number 8 is an important indicator of stability in chemistry, and understanding its significance can help us understand the behavior of different elements and compounds. The “2-8-8” rule states that the outermost shell of an atom can contain a maximum of 8 electrons, and this rule applies to the first 18 elements in the periodic table, from hydrogen to argon. We also know that two atoms can share 8 electrons in order to form a covalent bond.
What is the rule of 8 in atoms?
Atoms are the smallest particles that make up all matter. They are composed of protons, neutrons, and electrons. The octet rule is a principle in chemistry that helps us to understand how atoms interact with each other. It states that atoms are most stable when they have eight electrons in their outer shell, giving them the electron configuration of a noble gas. This rule helps us to predict the behavior of atoms in chemical reactions and to draw Lewis diagrams.
The octet rule is a chemical principle that states that atoms are at their most stable when they have eight electrons in their outer shell. This is because the outer shell is the most reactive shell and atoms want to be as stable as possible. When an atom has fewer than eight electrons, it will react and form more stable compounds. When discussing the octet rule, we do not consider d or f electrons.
How is the Octet Rule Used?
The octet rule is used to predict the behavior of atoms in chemical reactions. It helps us to draw Lewis diagrams, which are diagrams that show the electrons in a molecule. When assigning electrons to atoms, make sure that each atom has eight electrons in its outer shell. This will ensure that the atoms are stable.
Are there Exceptions to the Octet Rule?
Yes, there are some exceptions to the octet rule. Molecules with odd numbers of electrons disobey the octet rule. An example of this is Na7- and Cl7+, which is much less stable than Na+ and Cl-. Atoms are more stable when they have no charge, or a small charge.
The octet rule is a principle in chemistry that states that atoms are at their most stable when they have eight electrons in their outer shell. This rule helps us to predict the behavior of atoms in chemical reactions and to draw Lewis diagrams. There are some exceptions to the octet rule, such as molecules with odd numbers of electrons. Understanding the octet rule can help us to better understand chemical reactions and the behavior of atoms.
What is 8 electron rule?
The octet rule is a widely accepted principle in chemistry that states that atoms tend to form chemical bonds in such a way that they have eight valence electrons in their outermost shell. This is also known as the Abegg rule, after Richard Abegg, who proposed it in 1904. The octet rule is useful for predicting the stability of atoms and molecules and for understanding the reactivity of chemical compounds.
What are Valence Electrons?
Valence electrons are the outermost electrons of an atom. They are the electrons that are involved in forming chemical bonds with other atoms. The number of valence electrons an atom has can vary from one to eight. The octet rule states that atoms prefer to have eight valence electrons in their outermost shell.
What is the Rule for 8 Valence Electrons?
The octet rule states that atoms prefer to have eight valence electrons in their outermost shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. When discussing the octet rule, we do not consider d or f electrons. Only the s and p electrons are involved in the octet rule, making it useful for the main group elements (elements not in the transition metal or inner-transition metal blocks); an octet in these atoms corresponds to an electron configurations ending with (s^2p^6).
Why Do Atoms Prefer 8 Valence Electrons?
Atoms prefer to have eight valence electrons because it is the most stable electron configuration. It is the most stable because it has the maximum number of electrons in the outermost shell, which gives the atoms the greatest stability. An atom with eight electrons in its outermost shell is said to be in a “closed shell” configuration, which means it is least likely to react with other atoms.
What Are the Exceptions to the Octet Rule?
The octet rule is not always followed. Atoms such as hydrogen and helium, which have only one or two electrons in their outermost shell, can form molecules with fewer than eight electrons. Atoms in the transition metal and inner-transition metal blocks can also form molecules with more than eight electrons. This is because these atoms have empty d and f orbitals which can hold additional electrons.
In summary, the octet rule is a widely accepted principle in chemistry that states that atoms tend to form chemical bonds in such a way that they have eight valence electrons in their outermost shell. This rule is useful for predicting the stability of atoms and molecules and for understanding the reactivity of chemical compounds. The octet rule is not always followed, as atoms in the transition metal and inner-transition metal blocks can form molecules with more than eight electrons.
Why is the octet rule called the Rule of 8?
The octet rule is a fundamental principle in chemistry that states that atoms in a molecule tend to form bonds so that each atom has eight electrons in its outermost shell. This is why it is also known as the “Rule of 8.” It is a valuable tool for predicting the structure of molecules and the stability of ions.
In order to understand the octet rule, it is important to first understand the structure of an atom. Atoms are made up of a nucleus surrounded by electrons. The nucleus contains protons and neutrons, while the electrons are found in shells around the nucleus. The electrons in the outermost shell are called the valence electrons, and they are the ones that are involved in forming chemical bonds.
Limitations and Exceptions of the Octet Rule
Although the octet rule is a great model, it doesn’t always hold up. In fact, it has some notable exceptions. For example, some molecules have odd numbers of electrons, making it impossible for them to obey the octet rule. Examples of these molecules include free radicals such as nitric oxide and nitrogen dioxide.
In addition, some elements do not obey the octet rule because they are electron deficient or electron rich. Elements such as hydrogen, lithium, phosphorus, and sulfur are all examples of elements that do not obey the octet rule. These elements can form molecules that have fewer than eight or more than eight electrons in their outermost shell.
Finally, some molecules can be hypervalent, meaning that they have more than eight electrons in their outermost shell. Examples of hypervalent molecules include sulfur hexafluoride, phosphorus pentafluoride, and sulfur dioxide.
The octet rule is a great tool for predicting the structure of molecules and the stability of ions. However, it is important to remember that it is not applicable to all molecules and compounds. Some compounds are electron deficient while others are electron-rich, and some molecules are hypervalent. Therefore, it will be true to say that the octet rule is not universal.
By understanding the exceptions to the octet rule, it is possible to gain a better understanding of the structure of molecules and the stability of ions. This can help chemists and other scientists to better predict the behavior of molecules and ions in different situations. Additionally, it is a great tool for teaching students the fundamentals of chemistry.
Are there 8 valence electrons?
Valence electrons are the electrons in the outermost shell of an atom and play an important role in chemical bonding. But why is it that most elements have a maximum of 8 valence electrons? The answer lies in the structure of the atom.
Electron Shells and Orbitals
Atoms are made up of a nucleus (containing protons and neutrons) surrounded by a cloud of electrons. The electrons are organized into shells or energy levels. The first shell can hold a maximum of two electrons, the second shell can hold a maximum of eight electrons (2+6=8). This is known as the octet rule.
The number of electrons in each shell depends on the number of orbitals. Orbitals are regions in space where electrons are likely to be found. The first shell has one orbital (1s) and two electrons. The second shell has four orbitals (2s, 2p x , 2p y , 2p z ). Each orbital can hold two electrons. This means that the second shell can hold a maximum of eight electrons (2+6=8).
Valence Electrons and the Periodic Table
The periodic table is a chart that organizes elements according to their atomic number, which is the number of protons in an atom. Elements in the same group (column) have the same number of valence electrons. The group number indicates the number of valence electrons. Group 1 elements have one valence electron, Group 2 elements have two valence electrons, and so on.
In the electron dot structures used here, valence electrons are shown as dots. For example, Carbon (C) has four valence electrons. It is in Group 4, so it has four valence electrons.
It turns out that there is something special in nature about having eight electrons in the valence shell. This happens be the most stable situation for an atom. The elements that have this number in their pure neutral forms are the elements in Group VIII. These elements, which include oxygen and fluorine, have eight valence electrons and are called the noble gases.
Can an Atom Have 9 Valence Electrons?
An element never has more than eight valence electrons, so there can’t be more than eight dots per atom. However, some atoms can gain or lose electrons to become ions and can have more than eight valence electrons. For example, sodium (Na) has one valence electron. In the ionic form, Na +, it has lost that one electron and now has seven valence electrons. On the other hand, chlorine (Cl) has seven valence electrons. In the ionic form, Cl -, it has gained one electron and now has eight valence electrons.
Limitations of the Octet Rule
The octet rule is applicable only for atoms in their ground state. This means that it doesn’t apply to atoms that are in excited states, which can have more than eight valence electrons. The octet rule is also not applicable to molecules, which can have more than eight valence electrons.
In summary, there can only be eight valence electrons because of the structure of the atom. Representative elements in the same group have the same number of valence electrons. It turns out that there is something special in nature about having eight electrons in the valence shell. Atoms can gain or lose electrons to become ions and can have more than eight valence electrons. However, the octet rule has its limitations and is not applicable to all atoms and molecules.



Leave a Comment