Chemistry Behind Mixing Isopropyl Alcohol and Sodium Chloride
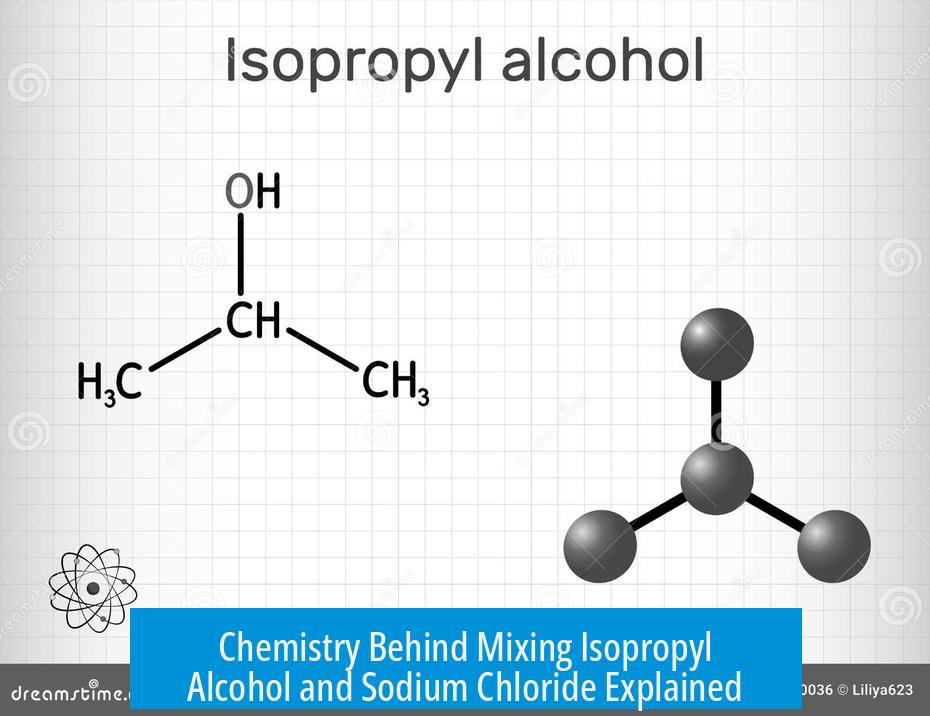
The chemistry behind mixing isopropyl alcohol and sodium chloride mainly involves physical processes rather than chemical reactions. Sodium chloride (NaCl) acts as a mechanical scrubbing agent, not a chemical reactant. Meanwhile, isopropyl alcohol serves as a solvent that dissolves oil-soluble compounds.
Role of Sodium Chloride
Sodium chloride functions primarily as a scrubbing tool. It helps physically remove residues such as sticky byproducts from glass surfaces without scratching them. This mechanical action aids in cleaning but does not involve chemical change.
- NaCl crystals provide gentle abrasion.
- Residues can be loosened and wiped away more easily.
- Salt is safe and easily discarded after use.
Isopropyl Alcohol as a Solvent
Isopropyl alcohol dissolves non-polar substances like oils and many organic compounds. It effectively dissolves residues such as THC and its byproducts, which are oil-soluble. The alcohol’s polarity allows it to break down these compounds for cleaning.
- THC byproducts are soluble in isopropyl alcohol.
- Alcohol disrupts and dissolves sticky residues.
- This promotes thorough cleaning of surfaces.
Salt-Induced Phase Separation
When sodium chloride is added to alcohol-water mixtures, it induces phase separation. The salt decreases the alcohol’s miscibility with water, allowing purer alcohol to separate and form a distinct layer. This physical effect can be useful for alcohol purification.
| Effect | Explanation |
|---|---|
| Salt reduces water solubility of alcohol | Salt ions bind water molecules, lowering alcohol-water miscibility |
| Phase separation occurs | Alcohol forms a purer, upper layer above water-salt solution |
| Impurities remain in lower layer | Allows careful extraction of purer alcohol fraction |
Chemical Interactions
There is limited evidence of specific chemical reactions between sodium chloride and isopropyl alcohol or THC byproducts. The main process involves physical dissolution and mechanical scrubbing. Any complex chemistry remains unclear or undocumented.
Salt Type Considerations
Rock salt and Himalayan salt mainly differ in mineral content but are chemically similar as NaCl. Both can serve the physical scrubbing role interchangeably with isopropyl alcohol.
Key Takeaways
- Sodium chloride acts primarily as a physical abrasive, not a chemical reactant.
- Isopropyl alcohol dissolves oil-soluble residues like THC byproducts.
- Salt addition to alcohol-water mixtures causes phase separation, aiding purification.
- No significant chemical reactions occur between NaCl and isopropyl alcohol.
- Different salts (rock, Himalayan) serve similar mechanical cleaning purposes.
What role does sodium chloride play when mixed with isopropyl alcohol?
Sodium chloride acts mainly as a physical scrubbing agent. It helps remove residues by abrasion without scratching surfaces like glass. It does not chemically react with isopropyl alcohol in this context.
How does isopropyl alcohol interact with substances dissolved in the mixture?
Isopropyl alcohol dissolves oil-soluble compounds, including THC and its byproducts. It works as a solvent to break down residues left behind from smoking or other sources.
Does sodium chloride chemically change the isopropyl alcohol or its dissolved components?
There is no clear evidence of chemical reactions between sodium chloride and isopropyl alcohol or THC byproducts. Sodium chloride mainly affects the physical properties like phase separation.
How does sodium chloride affect alcohol-water mixtures?
Sodium chloride causes phase separation by reducing alcohol’s solubility in water. This action can create a layer of purer alcohol on top after settling, which can be separated physically.
Can Himalayan salt be used instead of rock salt with isopropyl alcohol?
Himalayan salt and rock salt are both primarily sodium chloride. They function similarly in scrubbing and phase separation with isopropyl alcohol.




Leave a Comment