Polyurethane does not truly dissolve in any common solvent due to its highly cross-linked polymer network formed by bifunctional isocyanates and multifunctional cross-linkers like tetraethyl orthosilicate (TEOS). This cross-linking interconnects all polymer chains into a giant molecule, preventing dissolution. Instead, certain polar solvents can swell or soften polyurethane, easing mechanical removal.
Understanding Polyurethane Structure and Solubility
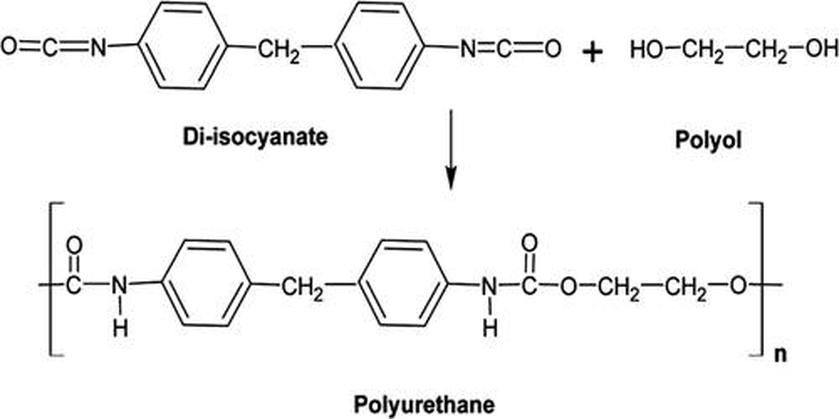
Polyurethane typically forms a three-dimensional, cross-linked polymer network during curing. The base reaction involves bifunctional isocyanates that polymerize end-to-end, forming long chains. When multifunctional cross-linkers such as TEOS join this reaction, they bond multiple chains together at network points.

This network means all polymer chains interconnect, creating a single massive molecule rather than discrete polymer chains. Such cross-linking gives polyurethane its strength, rigidity, and resistance to solvents. In analogy, one can pull a single thread out of a loose ball of yarn, but when the yarn is firmly knotted like a fisher’s net, single threads cannot be pulled free. Thus, polyurethane cannot be dissolved but may be swollen or softened.
Solvents That Soften or Swell Polyurethane

While true dissolution is not achievable for cured polyurethane, certain solvents can penetrate unlinked regions and cause swelling or softening. Given polyurethane’s somewhat polar nature, polar organic solvents are more effective than nonpolar ones like naphtha or xylenes.
- Acetone: A polar aprotic solvent capable of softening polyurethane with sufficient soak time. Its rapid evaporation can be mitigated by sealing the container during soaking.
- Ethyl Acetate: Another polar solvent that helps swell the polymer matrix, easing removal.
- Methyl Ethyl Ketone (MEK): Commonly used industrial solvent for softening polyurethane layers. It penetrates the network but does not dissolve it.
- Mineral Spirits: Can soften cured polyurethane foam, although less effective than acetone or MEK.
These solvents do not break the cross-linked bonds but merely swell or soften the polymer by penetrating non-cross-linked segments. They facilitate mechanical removal such as scraping or peeling.

Solvents for Uncured Polyurethane and Cleanup
Before polymerization, polyurethane components remain soluble. Traditional solvents like naphtha dissolve uncured isocyanates and polyols, enabling cleaning of tools and spills. Once cured, however, these solvents have minimal effect on the networked polymer.

Strong Chemical Agents: Not a Practical Solution
Highly reactive chemicals such as concentrated sulfuric acid, red fuming nitric acid, or piranha solution can chemically degrade polyurethane. While they may break down the polymer, their extreme corrosivity and hazardous nature make them impractical choices for routine polyurethane removal.

Mechanical Removal is Often Necessary for Cured Polyurethane
Because cured polyurethane resists dissolution, mechanical methods remain the preferred removal technique. Scraping, sanding, or cutting the material off surfaces is common practice. Solvent softening beforehand by acetone or MEK can ease this process.
Using non-stick silicone coatings on treated surfaces may prevent fresh polyurethane adhesion, facilitating future cleaning.
Household Method for Polyurethane Removal from Wood
An effective non-solvent method involves a three-part mixture:
- Murphy’s Oil Soap
- Paint stripping gel
- Water (to dilute the gel)
This mixture breaks down polyurethane films on wood. After application, an abrasive wood filler is scrubbed in, absorbing oils and exfoliating the surface. Subsequent rinsing with diluted solution and cool water causes the polyurethane to flake off in powder form, which can be collected easily. This process likely disrupts polyurethane’s adhesive properties by disrupting oils within the polymer matrix, encouraging separation.
Chemical Basis and Precursors Relevant to Solubility
Polyurethane synthesis involves two main components: bifunctional isocyanates and polyols. Cross-linkers such as TEOS add multifunctional linkages, connecting polymer chains at several points. TEOS reacts with isocyanate groups, creating three-dimensional networks responsible for solvent resistance.
The extensive cross-linking explains why solvents cannot dissolve cured polyurethane. Breaking down the polymer would require disrupting stable covalent bonds, a demanding chemical process rarely achieved with conventional solvents.
Summary Table: Solvent Effects on Polyurethane
| Solvent Type | Effect on Polyurethane | Common Uses | Limitations |
|---|---|---|---|
| Acetone | Swells/softens polymer network | Softening cured polyurethane before mechanical removal | Rapid evaporation; no true dissolution |
| Ethyl Acetate | Swelling agent | Softening for easier scraping | Slow action; requires soaking |
| Methyl Ethyl Ketone (MEK) | Swelling and softening | Industrial softening agent | No dissolution; requires handling precautions |
| Mineral Spirits | Minor softening effect | Cured foam softening | Less effective than polar solvents |
| Naphtha | Dissolves uncured components | Cleaning uncured parts and tools | Ineffective on cured polymer |
| Strong Acids (e.g. H2SO4) | Polymer degradation | Not recommended due to hazards | Corrosive, dangerous to handle |
Key Takeaways
- Polyurethane’s cross-linked polymer network prevents its dissolution by conventional solvents.
- Polar solvents such as acetone, ethyl acetate, and MEK soften and swell polyurethane but do not dissolve it.
- Uncured polyurethane parts dissolve in solvents like naphtha and are easier to clean during application.
- Mechanical removal techniques remain necessary for cured polyurethane.
- A household mixture of Murphy’s Oil Soap, paint stripper gel, and water combined with abrasive wood filler can effectively break down polyurethane on wood surfaces.
- Strong acids or aggressive chemicals can degrade polyurethane but pose safety risks.
- Preventive coatings like silicone can reduce future polyurethane adhesion, easing cleaning efforts.
What Solvent Will Best Dissolve My Polyurethane? Details on Specific Precursors in Description
Let’s cut to the chase — if you want to dissolve cured polyurethane, you’re in for a tough ride because it’s pretty much insoluble. Why? It’s all about chemistry and the unique structure of this polymer. But that doesn’t mean you’re stuck forever with sticky polyurethane. There are ways to soften, swell, and even chemically challenge the material, depending on whether it’s cured or not. Let’s dive into the nitty-gritty of what polyurethane is, why it behaves the way it does, and practical tips on what solvents might help—or fail miserably.
Understanding Polyurethane’s Insolubility
Think about polyurethane as one massive molecule rather than thousands of little ones. It’s not your average polymer chain lying around inviting solvents to dissolve it. Polyurethane features a cross-linked network, where multiple polymer chains get tightly bonded via special molecules:
- Bifunctional isocyanates – These connect two ends of polymer chains, like links in a chain.
- Multifunctional cross-linkers such as tetraethyl orthosilicate (TEOS) – These are the overachievers, connecting multiple chains together in all directions.
This network forms a three-dimensional, heavily knotted mesh. Imagine trying to pull apart a fisher’s net tightly tied, rather than pulling apart a loose ball of yarn. That’s essentially what makes polyurethane so stubborn against typical solvents.
Why Don’t Solvents Dissolve Polyurethane?
Solvents dissolve substances by interacting with individual molecules and breaking them apart from the mix. But the extensive cross-linking turns polyurethane into one giant molecule, where chains are locked together tightly. Solvents can slip between the chains only where there’s space and make the material swell but not truly dissolve it. They might soften the surface or the bulk somewhat, but you won’t get the goo magically turning into liquid.
So, while chemical dissolution is mostly a no-go, certain solvents can at least soften or swell the polymer, which is a useful starting point if you want to remove or scrape it off easier.
Recommended Solvents for Polyurethane Softening and Swelling
If outright dissolution is a lost cause, softening is a winner. Here’s the solvent shortlist:
- Acetone – A polar solvent known for vigorous cleaning abilities. It needs to soak on the polyurethane for quite a while — and sealed — so it doesn’t evaporate immediately.
- Ethyl Acetate – Another polar candidate; standing a chance to interact with and swell the polymer somewhat.
- Methyl Ethyl Ketone (MEK) – Similar to acetone, MEK works well for softening if you can soak the polyurethane.
- Mineral Spirits – Less polar but still useful for softening cured foam; won’t dissolve but can soften material on surfaces.
- Naphtha – Great for cleaning uncured components, given that polyurethane parts and precursors are usually dissolved in it.
Soak polyurethane in these polar solvents, and the outer layer will swell, making mechanical removal (scraping or sanding) more manageable. Patience is key. You might have to let it soak for hours, even overnight.
The Wild Side: Strong Acids and Harsh Chemicals
Here’s a chemistry stunt you probably won’t want to try at home: intense acids like concentrated sulfuric acid, red fuming nitric acid, or the infamous “piranha” solution (a mix of sulfuric acid and hydrogen peroxide) will certainly degrade polyurethane. But this approach is a hazardous mess involving fire risks, toxic fumes, and corrosion. Not exactly your friendly neighborhood cleaner! So unless you want a chemistry lab disaster, skip this option.
How Polyurethane Precursors Affect Solubility and Cleaning Approaches
Most polyurethanes are formed from a two-part reaction:
- A set of bifunctional isocyanates, which join chains end-to-end.
- Cross-linkers like TEOS, which bind chains together across multiple points creating the 3D network.
The presence and amount of these cross-linkers determine the hardness and insolubility. More TEOS means a denser network, less chance for any solvent to break it down. This explains why curing transforms polyurethane into something impervious to typical solvents.
So, What’s the Best Strategy for Removing Polyurethane?
If your polyurethane is already cured, don’t expect it to dissolve in simple solvents. Instead, use this three-tiered approach:
- Softening: Use polar solvents like acetone or MEK to swell the polyurethane. This weakens the network at the surface.
- Mechanical Removal: Scrape or sand off the softened material. A razor scraper, wire brush, or gentle sanding can do wonders once the polymer is softened.
- Surface Treatments: After removal, sealing the area with non-stick coatings (perhaps silicone-based) can prevent future adhesion issues.
For uncured polyurethane, or accidental spills before the polymer sets, naphtha and similar solvents are effective cleaners because they are the base for the uncured materials.
A Surprisingly Handy Household Hack for Polyurethane on Wood
If you’re battling polyurethane on wood and want to avoid harsh chemicals, here’s a homemade cleaning concoction to try:
- Mix Murphy’s Oil Soap (1 part)
- Paint stripping gel (a few parts)
- Water (to dilute the gel)
Apply this mix to the poly-covered wood. Let it sit until it becomes less gooey, then scrub with an abrasive medium like wood filler. The wood filler acts double-duty: it’s abrasive enough to assist in removing the film and absorbs the oily residues that make polyurethane sticky and hard to clean.
Rinse with a diluted version of the original mix and a splash of cool water. Watch the polyurethane start to flake off almost like powder! The powdery mess can be washed away easily with water, which prevents it from sticking back.
This hack works by “confusing” the polyurethane into separating itself, weakening its grip on the wood. Plus, the oil absorption by the wood filler cuts the stickiness. Not perfect yet (ratios need fine-tuning), but a great non-toxic alternative for delicate restoration work.
Practical Final Thoughts and Recommendations
- Expect that cured polyurethane won’t dissolve, but it will soften under prolonged solvent exposure.
- Use acetone, MEK, or ethyl acetate when you want to swell or soften polyurethane before scraping.
- Clean up uncured parts with naphtha to avoid permanent adhesion or hardening.
- Try the household soap-gel-water combo for polyurethane on wood if chemical solvents are off-limits.
- Avoid strong acids unless you’re a chemist or seek demolition rather than cleaning.
Why Does This Matter to You?
Polyurethane is everywhere: furniture, coatings, foams, adhesives, and more. Knowing how to remove or work with polyurethane can save you from frustration and costly damages. For contractors and DIYers, the key is patience and the right solvent choices backed by chemistry understanding.
Next time you stare at a stubborn polyurethane blob, remember: it’s not being rude; it’s just that it’s a complex, cross-linked giant molecule that doesn’t break down easily. But armed with the right solvents and methods, you can make that giant molecule soften up and eventually say goodbye.
| Solvent | Effect on Polyurethane | Best Use |
|---|---|---|
| Acetone | Swells and softens cured polyurethane | Soaking for long periods in a sealed container |
| Methyl Ethyl Ketone (MEK) | Similar to acetone; softens polymer | Soaking and surface preparation |
| Ethyl Acetate | Polar solvent that can swell polymer | Softening and cleaning surfaces |
| Mineral Spirits | Softens cured foam but does not dissolve | Surface softening of hardened foam |
| Naphtha | Dissolves uncured parts | Cleaning spills before curing |
| Strong Acids (e.g., sulfuric acid) | Can degrade polymer but hazardous | Not recommended for routine maintenance |
If you want to go deep into polyurethane chemistry and solvent science or share your personal experiences with these solvents, drop a comment below. Got a crazy polyurethane removal hack? Let’s hear it!
What type of polyurethane structure affects its solubility?
Polyurethane has a cross-linked structure. This means its polymer chains are tightly connected. Because of this network, it does not dissolve easily in solvents.
Can solvents fully dissolve cured polyurethane?
No. Solvents can swell or soften polyurethane by entering spaces between chains, but they cannot dissolve it. The cross-linking keeps the polymer intact and resistant to dissolution.
Which solvents are recommended to soften or swell polyurethane?
Polar solvents like acetone, ethyl acetate, and methyl ethyl ketone can soften polyurethane. They require long soaking times to penetrate and swell the material but won’t fully dissolve it.
Are strong acids effective solvents for polyurethane?
Strong acids such as sulfuric or nitric acid can destroy polyurethane. However, these are hazardous and not practical for typical applications or clean-up.
What solvent works best for cleaning uncured polyurethane parts?
Uncured parts dissolve in solvents like naphtha. Since the raw components are dissolved in naphtha, it is effective for cleaning before polymerization occurs.




Leave a Comment