What the Heck Is Enthalpy?

Enthalpy is the total heat energy of a system, representing its internal energy plus the energy required to establish its physical state at constant pressure. It plays a crucial role in chemistry and physics, especially when studying reactions and phase changes.
Definition of Enthalpy
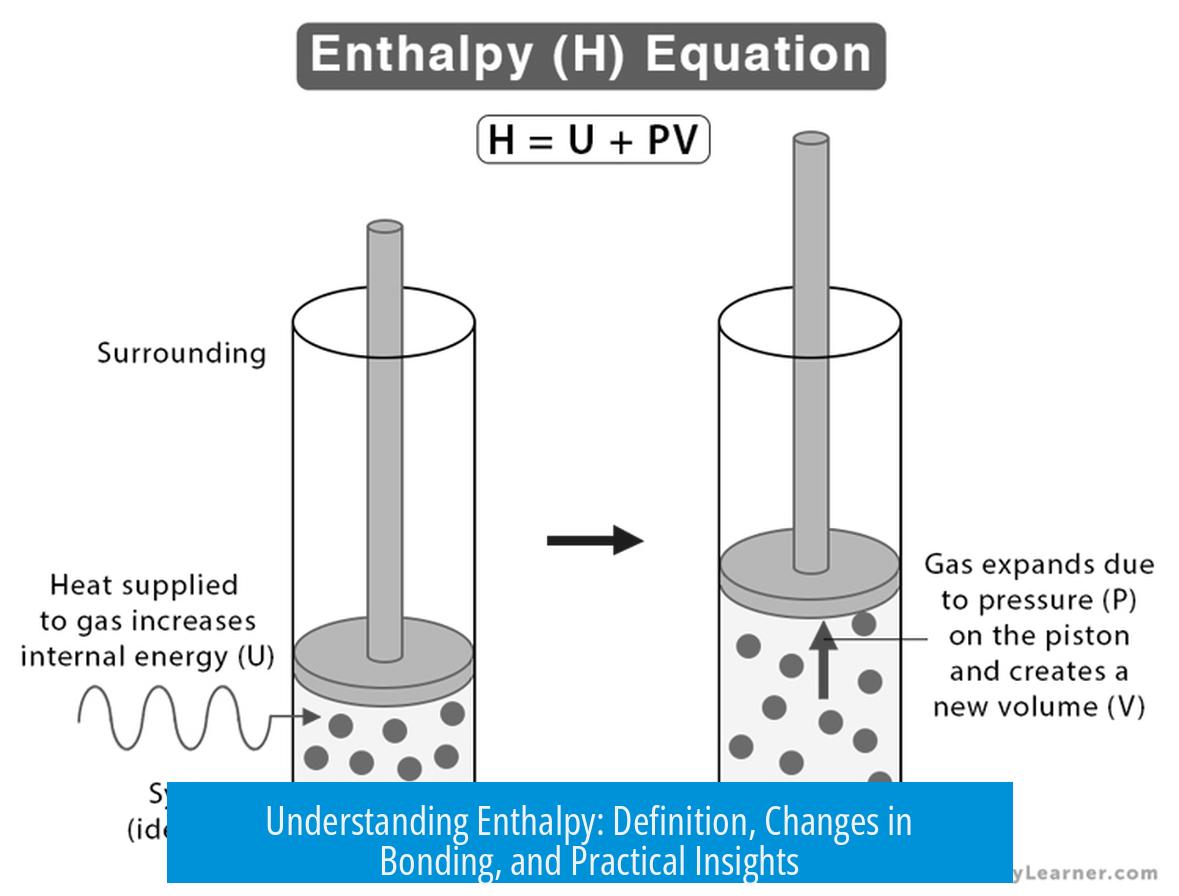
Enthalpy (H) combines the system’s internal energy (E) and the pressure-volume work (PV) needed to create its physical boundaries:
H = E + PV
If pressure (P) is constant and very low, the PV term becomes negligible. Then, enthalpy closely approximates internal energy and heat exchange (q) at constant pressure:
H ≈ E ≈ q
Molecular-Level View
At the molecular scale, enthalpy includes energy stored in chemical bonds and intermolecular forces. Attractive forces between atoms and molecules build potential energy.
Kinetic energy of moving atoms or molecules also contributes, although for solids and liquids this part is often small. Enthalpy reflects the system’s total energetic state.
Enthalpy Changes in Bonding
- When atoms form a bond, enthalpy decreases and heat is released at constant pressure.
- When bonds or intermolecular forces break, enthalpy increases and heat is absorbed.
These energy changes affect reaction spontaneity and heat flow during physical transformations.
Energetic Effects Inside Enthalpy
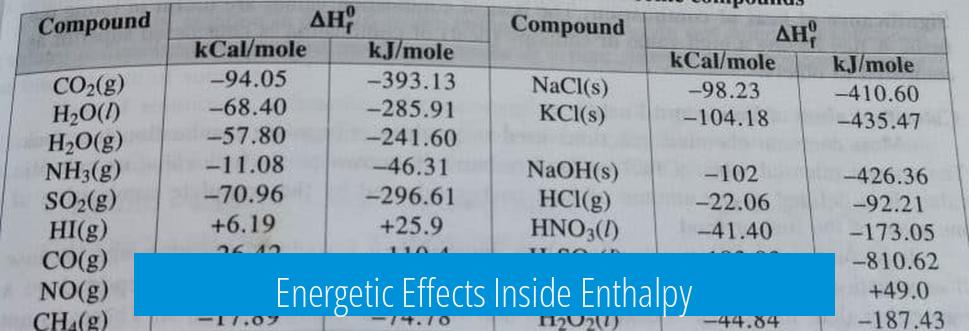
Enthalpy arises from electronic interactions like electron-electron repulsions, electron-nucleus attractions, and orbital overlaps. These effects define the material’s energetic landscape.
Advanced Context
Exploring enthalpy fully requires condensed matter physics, where material phases and quantum states are studied. Looking at the eigenstates of the Hamiltonian operator for a substance reveals underlying conservation laws affecting enthalpy.
Practical and Simulation Insights
In simulations, enthalpy combines potential energy (due to molecular attraction) and kinetic energy. It tends to be negative for condensed phases and positive for gases, reflecting stability and phase behavior.
Key Takeaways
- Enthalpy = internal energy + pressure-volume energy at constant pressure.
- It includes chemical bond energy and molecular forces.
- Bond formation lowers enthalpy, bond breaking raises it.
- Electronic forces contribute to enthalpy’s energy.
- Advanced study links enthalpy to quantum states and material phases.
- Simulations treat enthalpy as sum of potential and kinetic energies.
What the heck is enthalpy? Let’s dive in!

Enthalpy is basically the total heat energy of a system. That’s the simplest way to get your head around it without wandering off into a maze of equations and jargon. When chemists or physicists talk about enthalpy, they’re referring to the energy stored and used during chemical reactions or physical changes — all under constant pressure.
So, when you see the letter H in your textbook, that’s enthalpy. But how does this work exactly? Let’s break it down.
Think of a system’s internal energy as E. This is energy tied up in everything inside your little ‘scientific universe.’ And then there’s PV — the pressure-volume work needed to carve out space for this system and push back its surroundings. So in mathematical terms, H = E + PV.
Here’s a useful tip: when pressure (P) remains constant and is quite small, PV shrinks to insignificance. That means enthalpy H almost equals internal energy E, which equals the heat exchanged q. Simple, right?
What’s enthalpy at the microscopic level?
This is where things get a bit cozy — and molecular! Enthalpy hangs out in the forces pulling atoms and molecules together. Imagine these as tiny tug-of-war battles of attraction inside your system.
You can safely imagine enthalpy as the **potential energy** stored in those invisible strings binding atoms through intermolecular forces and chemical bonds. Plus, it includes the **kinetic energy** due to atoms zooming about.
But here’s a secret: When it comes to solids or liquids, the kinetic part is often tiny enough to ignore. Just think of it as potential energy having the starring role in enthalpy’s story.
Enthalpy in action: forming and breaking bonds
This part is all about giving and receiving heat. When atoms get together and form a bond, enthalpy dips. That means the system releases heat — a cozy warm feeling under constant pressure.
On the flip side, breaking bonds is like turning the heat back on. Energy flows into the system, increasing enthalpy as atoms move apart. So bond-breaking demands heat absorption.
In essence, enthalpy changes are the currency of heat transactions during molecular makeovers.
What makes up enthalpy energetically?
At the core, enthalpy arises from interactions like electrons repelling each other, electrons getting cozy with nuclei, and orbitals overlapping like puzzle pieces.
Unlike entropy, which deals with disorder and randomness — a purely statistical sort — enthalpy struts its stuff with these electron relationships and energetic interactions.
For those who want the nitty-gritty (Warning: it’s physics-heavy!)
Fair warning, enthalpy’s full story belongs in the realm of condensed matter physics. That’s where scientists explore how different phases of matter behave under various conditions.
If you’re craving to dive deeper, check out how quantum states, or eigenstates of the system’s Hamiltonian, come into play. In simple talk, the Hamiltonian helps predict how energy is conserved and transformed inside the material world.
How do professionals and simulations view enthalpy?
In practical fields, like chemistry or materials science, professionals just see enthalpy as a sum of potential energy (from molecular attraction) and kinetic energy (from molecular motion).
In simulations, things get interesting: enthalpy shows up as negative for condensed phases (think solids and liquids) and positive for gases. This difference tells a lot about how molecules stick together or fly apart.
Why should you even care about enthalpy?
Besides sounding cool, knowing about enthalpy helps in understanding why ice melts, why your coffee cools, and how engines work. It’s behind the scenes in fireworks, cooking, and even weather phenomena.
As a practical example, imagine heating water. You’re adding heat to the system, increasing enthalpy. When the water boils and turns to steam, the energy changes again — it’s not just about temperature but also how molecules interact.
If you’re running chemical reactions, measuring enthalpy change tells you if a process releases heat (exothermic) or sucks heat in (endothermic). This is crucial for designing everything from pharmaceuticals to fuels.
Quick tips to wrap your head around enthalpy:
- Focus on energy changes during chemical reactions at constant pressure.
- Remember, enthalpy includes both stored (potential) and moving (kinetic) energy.
- Bond-making cools the system (releases heat); bond-breaking warms it (absorbs heat).
- In condensed phases, enthalpy is usually negative; in gases, it turns positive.
- For deep dives, explore condensed matter physics and Hamiltonian mechanics.
So, next time you hear “enthalpy,” picture it as the system’s total heat, adjusting as molecules bond and part ways, revealing nature’s energy dance.
Isn’t it fascinating how such an invisible energy shapes the very world we live in?
What exactly does enthalpy measure in a system?
Enthalpy measures the total heat energy of a system. It combines internal energy and the work done to change the system’s volume.
How does enthalpy change when bonds form or break?
When atoms bond, enthalpy drops and heat is released. Breaking bonds raises enthalpy and absorbs heat, assuming constant pressure.
What role do molecular forces play in enthalpy?
Enthalpy includes energy stored in bonds and intermolecular forces. It also accounts for the motion energy of molecules, especially in gases.
Why is enthalpy important in phase changes or chemical reactions?
It tracks energy shifts from breaking or forming bonds and molecular attractions. This helps explain heat absorption or release in these processes.
How do scientists view enthalpy in simulations?
Enthalpy is the sum of potential and kinetic energies. For condensed phases, it often appears negative, while gases show positive values.




Leave a Comment