Difference Between NaCl and ClNa
NaCl and ClNa represent the same chemical substances: sodium and chlorine ions. However, only NaCl is the correct and accepted chemical formula. ClNa is not used because it violates chemical naming conventions.
Why NaCl Is Correct
Ionic compounds like sodium chloride consist of metal and nonmetal ions. Sodium (Na) is a metal, while chlorine (Cl) is a nonmetal. The chemical formula always places the metal symbol first, followed by the nonmetal. This rule ensures clarity and consistency in chemical communication.
Incorrect Formula: ClNa
Writing ClNa reverses this order. Although it contains the same elements, it does not follow accepted conventions and can cause confusion. Chemists and textbooks strictly use NaCl when referring to sodium chloride.
Proper Naming: Sodium Chloride, Not Sodium Chlorine
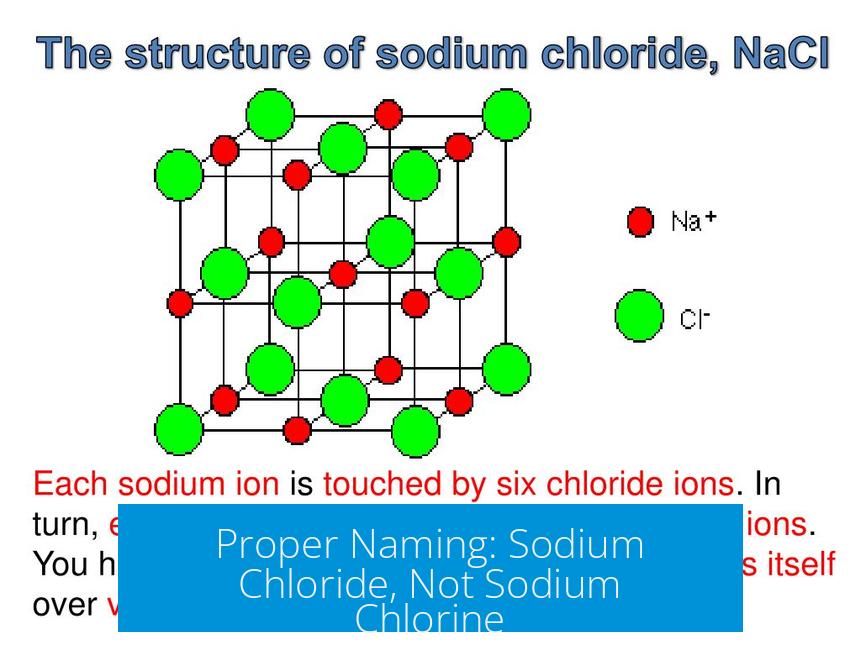
The compound is correctly named sodium chloride. This reflects the ionic form of chlorine as chloride (Cl−). Using “chlorine” implies the neutral element, which is different from the ionic compound.
Importance of Order in Chemical Formulas
Ordering in chemical formulas is like placing words correctly to convey meaning. For example, “anonymous 6561” differs from “6561 anonymous.” Both contain the same words, but only one is correct. Similarly, NaCl properly represents sodium chloride.
Summary of Key Points
- NaCl is the correct chemical formula for sodium chloride; ClNa is incorrect.
- The metal symbol (Na) always precedes the nonmetal (Cl) in ionic compounds.
- The compound name is sodium chloride, reflecting ionic species.
- Formula order follows international chemical naming conventions for clarity.
What’s the Difference Between NaCl and ClNa? They’re Both Sodium Chlorine, Salt, Right?

Let’s settle this: NaCl and ClNa represent the same chemical ingredients—sodium and chlorine—but only NaCl is the correct way to write it. ClNa? That’s just plain wrong in chemical speak.
If you ever caught yourself thinking, “Hey, salt is salt—does it really matter if we say NaCl or ClNa?” you’re on the right path but missing the chemistry fine print. This post dives deep into why conventions matter in chemistry, especially for something as common as table salt.
Naming Conventions: Chemistry’s Style Guide
In chemistry, writing formulas isn’t a free-for-all. There’s a widely accepted rulebook. The metal always comes first, then the nonmetal. Sodium (Na) is a metal. Chlorine (Cl) is a nonmetal. Therefore, sodium chloride is always NaCl, never ClNa.
Think of it like a proper name—your first name followed by your last name. You usually don’t say “Smith John” if your name is John Smith, right? Same principle here. Chemistry demands that order, so everyone stays on the same page.
Why the Order Matters in Ionic Compounds
It’s not just style; it’s meaningful. Ionic compounds are composed of metals and nonmetals. The metal donates electrons, becoming a positive ion (cation), and the nonmetal accepts electrons, becoming a negative ion (anion). By convention, chemists jot down the cation before the anion in formulas.
This means sodium, a metal and cation (Na+), always leads, and chlorine, the nonmetal and anion (Cl−), follows, resulting in NaCl. Writing ClNa would imply chlorine is leading — which defies the established ionic naming and is confusing.
It’s Not Sodium Chlorine, It’s Sodium Chloride

So, you might call it “salt” or “sodium chloride,” but not “sodium chlorine.” Why? Because chlorine and chloride are chemically different. Chlorine (Cl) is an element, a gas at room temperature, and quite reactive. Chloride (Cl−) is the negative ion form chlorine takes when it gains an electron—exactly what happens in table salt.
Calling NaCl “sodium chlorine” misses the ion aspect. “Sodium chloride” accurately reflects the ions present in the compound—a sodium ion and a chloride ion combined in equal parts.
Analogies to Cement the Concept
Imagine you have a buddy named “Anonymous 6561.” You wouldn’t write “6561 Anonymous” and expect people to understand properly, even though it’s composed of the same words. The order clarifies who or what you mean. Same with NaCl and ClNa; both have sodium and chlorine, but only one follows the accepted format, making it universally understandable.
Practical Implications: Why Care About NaCl vs. ClNa?
You might wonder: if it’s the same stuff, why complicate things? Scientists, educators, and industry professionals rely on consistent names to communicate clearly.
- Medical settings: Precise chemical names avoid dangerous mix-ups in prescriptions.
- Industrial manufacturing: Accurate compound formulas prevent costly errors in chemical processes.
- Education: Students learning chemistry gain clarity and avoid confusion.
Imagine a chemistry student writing ClNa in their exam. Even if they know the constituents, that slip might cost points. Consistency builds trust and ensures safety at scale.
Fun Fact: Table Salt in the Wild
You use NaCl every day without thinking. The salt shaker on your dining table is almost pure NaCl. Its crystal lattice forms due to the electrostatic attraction between Na+ and Cl− ions. Imagine the budding romance of opposites attracting and locking in place! This perfect lattice makes salt stable, easy to handle, and tasty.
Expert Tips: Remembering the Order
Next time you write about salt or ionic compounds, ask yourself: “Who’s the metal, who’s the nonmetal?”
- Identify metal (cation) first—usually on the left side of the periodic table.
- Write metal symbol first; then write nonmetal symbol.
- Use correct suffixes: for anions in ionic compounds, use “-ide” (chloride, oxide).
Keeping this checklist in mind saves headaches later and ensures chemical formulas are spot on.
Summary: NaCl vs. ClNa — More Than Just Letters
Though both NaCl and ClNa contain the same elements—sodium and chlorine—only NaCl follows chemical conventions and is the correct representation of table salt, sodium chloride.
The formula order respects the metal-first rule, clarifies the ionic nature with proper ion naming, and maintains universal communication standards. Skipping these rules turns straightforward salt into confusing jargon, much like mixing up first and last names.
Next time you sprinkle salt on your fries or read a chemistry textbook, appreciate the little details behind that humble NaCl formula. It’s not just letters—it’s a language with meaning.
Why is NaCl written instead of ClNa?
NaCl follows the naming conventions in chemistry. The metal, sodium (Na), is always written before the nonmetal, chlorine (Cl). ClNa is not correct because it reverses this order.
Are NaCl and ClNa the same compound?
Yes, they contain the same elements and ions: sodium and chlorine. However, only NaCl uses the accepted formula order for ionic compounds.
Is “sodium chlorine” the correct name for NaCl?
No. The proper name is sodium chloride. “Chloride” refers to the ionic form (Cl⁻), which is accurate in naming ionic compounds.
Does the order of elements in a formula matter?
Yes, order matters due to naming rules. Writing ClNa instead of NaCl is like swapping words in a name; it changes how the formula is understood and recognized.




Leave a Comment