Why Are We Still Taught That Secondary Alkyl Halides Can Undergo Both SN1 and SN2 Reactions?

Secondary alkyl halides have the distinct characteristic of being able to follow both SN1 and SN2 mechanisms, depending on various factors such as nucleophile strength, solvent type, and reaction conditions. This dual possibility remains a foundational concept in organic chemistry education due to the borderline nature of secondary alkyl halides in substitution reactions.
1. The Borderline Reactivity of Secondary Alkyl Halides
Secondary (2°) alkyl halides occupy an intermediate reactivity position. They do not strongly favor SN1 or SN2 exclusively but lean towards one or the other based on environmental and molecular factors. This ambiguity demands that students understand both mechanisms as possible pathways.
- The carbon skeleton influences stability and reaction route.
- The nature of the nucleophile—whether strong or weak—modulates mechanism preference.
- Solvent polarity and type (protic vs. aprotic) affect carbocation formation and nucleophile attack.
- The leaving group’s properties influence how readily substitution occurs.
Such a mix creates a competition between SN1 and SN2 pathways, making it important to teach that both can occur.
2. Influence of Nucleophile Strength and Solvent
2.1 Weak Nucleophiles in Protic Solvents Favor SN1/E1
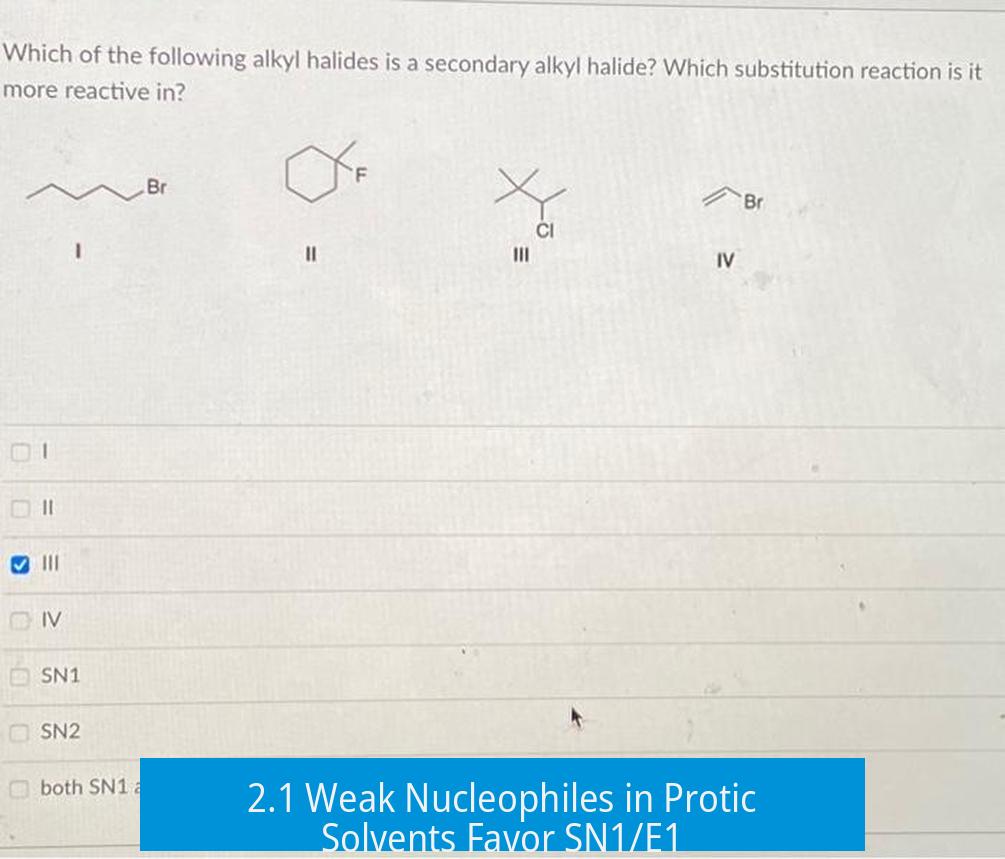
Secondary alkyl halides with weak nucleophiles, especially in protic solvents, may preferentially undergo SN1 or E1 reactions. Protic solvents stabilize carbocations and the leaving group, facilitating carbocation intermediate formation required for SN1.
- Secondary allylic and benzylic halides more readily undergo SN1/E1 under these conditions.
- Weakly basic nucleophiles do not promote direct displacement but allow carbocation pathways.
2.2 Weak Nucleophiles in Polar Aprotic Solvents Favor SN2
When weak nucleophiles are in polar aprotic solvents, the solvent does not stabilize carbocations, reducing SN1 feasibility. Instead, SN2 dominates by backside attack.
- Polar aprotic solvents enhance nucleophile strength by not hydrogen bonding.
- SN2 is favored with strong nucleophiles and weak bases in polar aprotic media.
2.3 Strong Nucleophiles and Bases Favor SN2 or E2
Strong nucleophiles, especially those that are weak bases, favor SN2 on secondary halides. Conversely, strong bases typically direct elimination via E2 rather than substitution.
- Secondary alkyl halides with strong, weakly basic nucleophiles proceed through SN2.
- E2 occurs when the nucleophile/base is strong and negatively charged.
3. Contemporary Research and Debate on SN1 Involvement
Recent studies have questioned the classical teaching that secondary alkyl halides readily undergo SN1 reactions. One 2009 paper argues the absence of SN1 pathways for secondary alkyl halides within specific conditions.
- Research focusing on solvolysis shows SN2 dominates substitutions in many cases.
- Neutral nucleophiles like triphenylphosphine (PPh3) perform SN2 on some secondary halides, even where SN1 was expected.
- Strong bases are needed for eliminations via E2; weakly basic negatively charged nucleophiles do not promote E2.
However, this research often concentrates on specific reaction conditions, such as highly nucleophilic environments, possibly skewing the observed mechanisms.
4. Limitations of Traditional Teaching Versus Research Findings
There is a tension between standardized teaching and cutting-edge research. Educational materials aim to present fundamental concepts comprehensively and simply, while actual research explores specific mechanistic nuances.
Teaching must standardize mechanisms like SN1 and SN2 for clarity.
Research delves into exceptions and detailed mechanistic pathways.
In real-world lab conditions, reactions often deviate from textbook norms due to subtle kinetic and environmental factors.
- Solvolysis reactions typically involve high nucleophile concentrations, shifting the balance toward SN2.
- Stoichiometric nucleophile concentrations can lead to rearrangements during substitution, characteristic of SN1 pathways.
- Secondary alkyl halides sometimes rearrange, indicating carbocation intermediates and thus SN1 involvement.
- If SN1 is impossible, corresponding elimination via E1 is also unlikely, impacting predicted reaction outcomes.
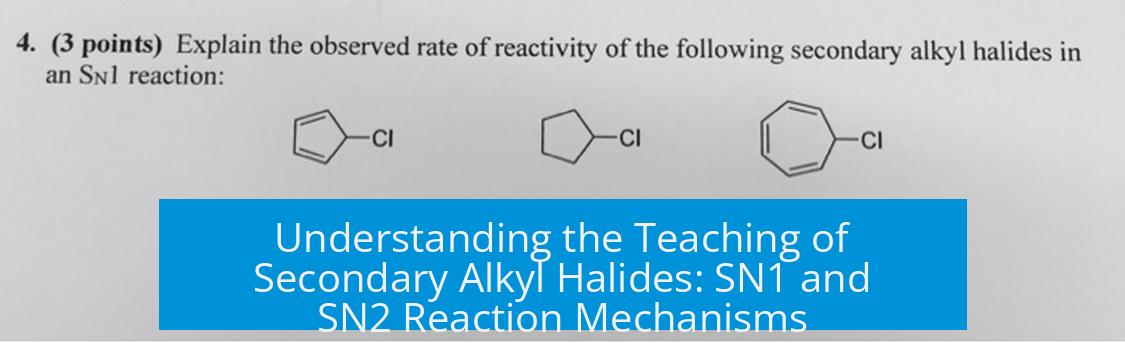
5. Nuanced Mechanistic Insights: Stereochemistry and Reaction Kinetics
Secondary alkyl halides exhibit interesting stereochemical and kinetic behavior in substitution reactions.
- Standard SN1 reactions typically produce racemization due to carbocation intermediates.
- However, some SN1 reactions may retain stereochemistry partially or fully, contrary to initial assumptions.
- Kinetics of some SN1 processes deviate from simple unimolecular models, revealing complex intermediates or concerted steps.
- Fully invertive SN1 reactions have been shown at tertiary centers (e.g., solvolysis with TMSCN), inviting further research on secondary centers.
6. Reflection on Scientific Dogma and Evolving Understanding
The ongoing debate illustrates the dynamic nature of science. What is taught as dogma often represents a consensus formed from decades of research. Yet, consensus can evolve or even be overturned.
- New data from advanced techniques contribute to nuanced views on reaction mechanisms.
- Careful interpretation of experimental findings is necessary before revising foundational concepts.
- Extreme caution is required when making sweeping claims about mechanisms without full data consideration.
- Teaching balances foundational understanding and openness to mechanism complexity.
Summary of Key Points
- Secondary alkyl halides can undergo both SN1 and SN2 reactions depending on reaction conditions.
- The nucleophile’s strength and solvent type are critical in determining the mechanism.
- Strong nucleophiles in polar aprotic solvents favor SN2; weak nucleophiles in protic solvents may allow SN1/E1.
- Recent research challenges some SN1 pathways for secondary halides but is context-dependent.
- Real-world reactions exhibit complexity beyond textbook mechanisms, including stereochemical nuances.
- Teaching includes both pathways to reflect the mechanistic complexity and variability accurately.
Why Are We Still Taught that Secondary Alkyl Halides Can Undergo Both SN1 and SN2 Reactions?
Let’s get straight to it: Secondary alkyl halides can indeed undergo both SN1 and SN2 reactions, but the actual pathway depends on a delicate balance of factors. It’s a classic case of chemistry refusing to be boxed into neat categories.
So, if you’ve ever scratched your head over why textbooks endlessly hammer home that secondary alkyl halides are switch-hitters—capable of running both SN1 and SN2 bases—you’re not alone. Let’s unpack the why, the how, and the ‘wait, seriously?’
Secondary Alkyl Halides: The Middle Child of Organic Chemistry
First, picture secondary alkyl halides as the ‘middle children’ in the alkyl halide family. They don’t lean as heavily towards SN1 as tertiary halides do, nor do they favor SN2 as strictly as primary halides. This middle-of-the-road status means their fate during substitution reactions is largely choreographed by the reaction environment.
Classic teachings impart this dual nature because, well, it’s the simplest way to introduce students to substitution reactions. “Here’s a rule of thumb,” they say, “remember it well: primary favors SN2, tertiary favors SN1, and secondary can party with both.” It’s tidy, memorable, and fits neatly onto exam cheat sheets.
But Is It That Simple in Reality?
Not quite. Recent research, especially from groups like MacMillan’s, has blown some of these textbook dogmas wide open. A 2009 paper claims that for secondary alkyl halides, SN1 might be less involved than previously thought, suggesting most substitutions lean on SN2 pathways. But—and here’s the kicker—their focus is largely on solvolysis reactions where nucleophiles are present in massive excess.
Why does this matter? Because having a high concentration of nucleophile isn’t just wildlife abundance, it’s a game changer. It tips the scale heavily in favor of SN2, which thrives when nucleophiles are eager and plentiful. Imagine a crowded dance floor—you have no excuse not to dance (SN2) when the music’s pumping and people keep inviting you.
What About the Experimental Evidence for SN1? The Carbocation Clues
Despite these claims, it’s far from settled that SN1 pathways vanish for secondary alkyl halides. Consider this: some reactions show evident signs of carbocation intermediates, like rearrangements during nucleophilic substitution. Carbocation rearrangements are hallmark SN1 signals because they need that intermediate to “do their thing.”
Therefore, reactions displaying rearrangement strongly suggest SN1 involvement. You can’t fake a molecular rearrangement—it’s akin to leaving a dance floor and returning with a different dance partner. That’s bona fide SN1 territory.
Don’t Forget Solvent and Nucleophile: The Unsung Puppeteers
It’s not just the halide; the solvent, nucleophile strength, and leaving group dramatically influence whether SN1 or SN2 fires up.
- Polar protic solvents—like water or alcohols—stabilize carbocations, helping SN1 proceed.
- Polar aprotic solvents—think DMSO or acetone—don’t stabilize carbocations as well but boost nucleophile strength, favoring SN2.
- Strong nucleophiles (like OH-) push SN2; weak ones (like water acting as nucleophile in solvolysis) subtly encourage SN1.
Secondary alkyl halides with allylic or benzylic positions can be extra tricky, as these resonance-stabilized carbocations sometimes make SN1 the easier path. So, the variability remains huge.
And What About Stereochemistry? Isn’t SN1 Always Racemization?
Here’s a shocker: SN1 doesn’t always behave like the textbook says. It’s often taught that SN1 equals racemization because of a planar carbocation intermediate, but new research finds SN1 can sometimes *retain* stereochemical configurations. There’s even evidence from tertiary centers where solvolysis leads to complete inversion via SN1—a twist that complicates the simplistic “SN1 equals racemization” mantra.
So, if SN1 pathways can sometimes mimic SN2 stereochemical outcomes (inversion), can we simplify this mechanism teaching further? Not easily. Chemistry likes its exceptions.
What About Elimination Reactions? The SN1 & E1 Domino Effect
Here’s a pragmatic nugget: If SN1 can’t happen, that undercuts E1 elimination too. Both SN1 and E1 share a carbocation intermediate. So, when people argue that secondary alkyl halides solely undergo E2 elimination (which requires a strong base), it’s often tied to rejecting SN1 possibility. However, the interplay complicates mechanistic assignments.
For example, weakly basic nucleophiles rarely trigger E2. Elimination pathways involving E1 or its cousin E1cB depend heavily on substrate features like adjacent carbonyls or leaving group placement. Secondary substrates, thus, sit at the crossroads of diverse mechanistic outcomes.
Why Is This Important to Learn? Why Do Textbooks Stick to the Old Story?
Let’s step away from theory and into practicality. Teaching is about building a foundation. Complex mechanism subtleties can perplex beginners. Educators choose consensus models that emphasize graspable concepts over cutting-edge nuance. This approach isn’t lazy; it’s pragmatic.
Still, scientific dogma is *not* gospel. Many experts caution that mechanistic debates continue. While consensus exists, it reflects best understanding so far, with active research refining details. Jumping to declare “SN1 for secondary alkyl halides doesn’t exist” based on one study is like ignoring decades of biochemical research because you just found one contradictory experiment.
Tips for Students and Chemists Navigating This Gray Zone
- Consider Reaction Conditions Carefully: Check solvent, nucleophile strength, and concentration before assuming the mechanism.
- Don’t Rely Solely on Textbook Rules: Use them as starting guidelines, not unchallengeable laws.
- Look for Experimental Clues: Rearrangements point to SN1; stereochemistry (inversion vs. retention) can hint at the pathway.
- Keep Updated With Literature: Research evolves; mechanisms once thought clear might have twists, especially for borderline cases.
- Remember the Big Picture: Mechanisms are models helping us predict outcomes, not rigid truths etched in stone.
Summary: The Practical Truth
We still teach that secondary alkyl halides can undergo both SN1 and SN2 reactions because this reflects the broad reality experienced in labs worldwide. The actual pathway is a tug-of-war influenced by nucleophile nature, solvent, temperature, and substrate specifics.
While recent research nuances or challenges conventional wisdom—particularly about universal SN2 dominance in solvolysis—it hasn’t outright refuted the possibility or prevalence of SN1 mechanisms under suitable conditions. Chemistry rarely offers pure black-or-white answers; instead, it thrives in gray areas filled with fascinating exceptions and exceptions to exceptions.
So, next time you see your secondary alkyl halide debating whether to go SN1 or SN2 on you, remember: It’s not indecisiveness. It’s chemistry being wonderfully complex—and that’s why you’re still learning both.
Why do secondary alkyl halides undergo both SN1 and SN2 reactions?
Secondary alkyl halides are borderline cases. The reaction path depends on factors like the nucleophile strength, solvent type, and leaving group nature. They can follow SN1 or SN2 based on the reaction conditions.
How does the nucleophile affect the mechanism for secondary alkyl halides?
Strong nucleophiles in polar aprotic solvents favor SN2. Weak nucleophiles in protic solvents promote SN1. The nucleophile’s basicity and solvent polarity play key roles in determining the pathway.
Is SN1 actually involved in reactions of secondary alkyl halides?
There is debate. Some research suggests SN1 may not occur, especially in solvolysis. However, rearrangements seen in some reactions support SN1 mechanisms with stoichiometric nucleophiles.
Why is the traditional teaching of both SN1 and SN2 mechanisms still used?
Teaching emphasizes standardized learning of mechanisms. Research provides nuanced views, but education aims to give foundational understanding, covering both pathways for common conditions.
Can SN1 reactions retain stereochemistry in secondary alkyl halides?
SN1 often leads to racemization, but partial stereochemical retention can occur. There are examples at tertiary centers showing fully invertive SN1; similar behavior might be possible in some secondary cases.



Leave a Comment