Why Does Electronegativity Increase Across and Up the Periodic Table?
Electronegativity increases across a period (left to right) and up a group (bottom to top) due to changes in nuclear charge, atomic size, and electron shell structure. These factors control how strongly an atom attracts electrons in a chemical bond.
Increasing Nuclear Charge Across a Period
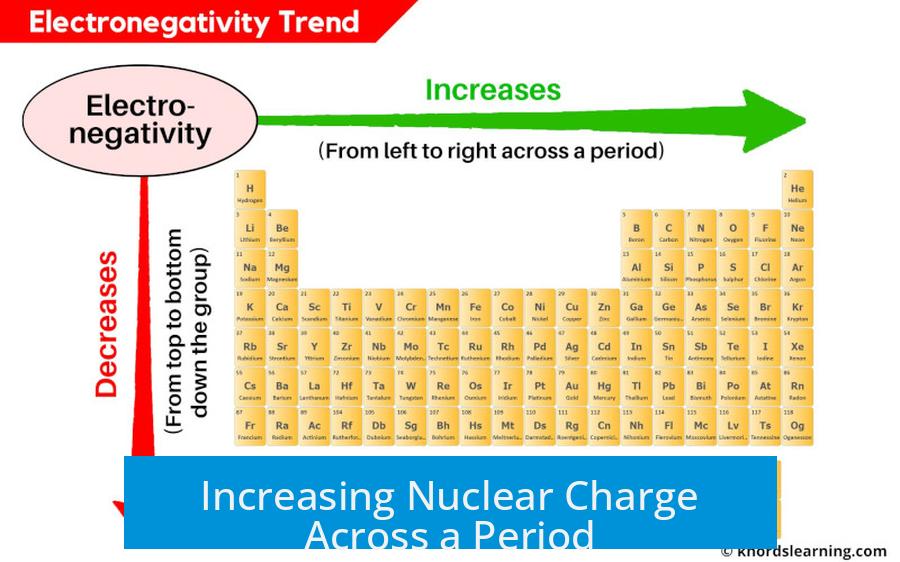
Moving across a period, atoms gain more protons in their nuclei. This higher positive charge pulls electrons more tightly. The increased attraction enhances the atom’s ability to attract electrons from other atoms. In simple terms:
- More protons → Stronger positive charge
- Stronger charge → Greater pull on electrons
- Greater pull → Higher electronegativity
Atomic Size Decreases Across a Period
As nuclear charge rises, electrons are pulled closer to the nucleus. This reduction in atom size means the outer electrons lie nearer to the nucleus. Since electric forces depend on distance, a smaller radius boosts attraction. The close proximity strengthens the pull on bonding electrons, raising electronegativity further.
Effect of Electron Layers Down a Group
Going down a group adds electron shells, increasing the distance between outer electrons and the nucleus. More layers create greater shielding, which weakens the nucleus’ pull on outer electrons. A larger atomic radius means the attraction for bonding electrons decreases, lowering electronegativity.
Filling of Electron Shells and Desire for Stability
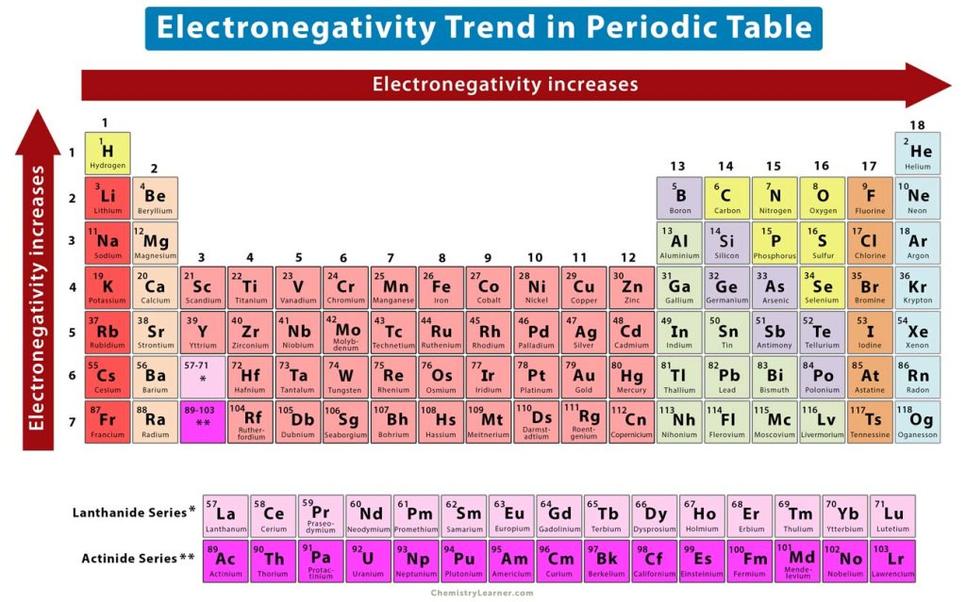
An atom’s tendency to attract electrons also depends on how full its outer electron shell is. Atoms near completing their outer shell strongly attract electrons to achieve stability. This effect grows stronger moving from left to right and bottom to top, complementing the influence of nuclear charge and size.
Summary of Factors
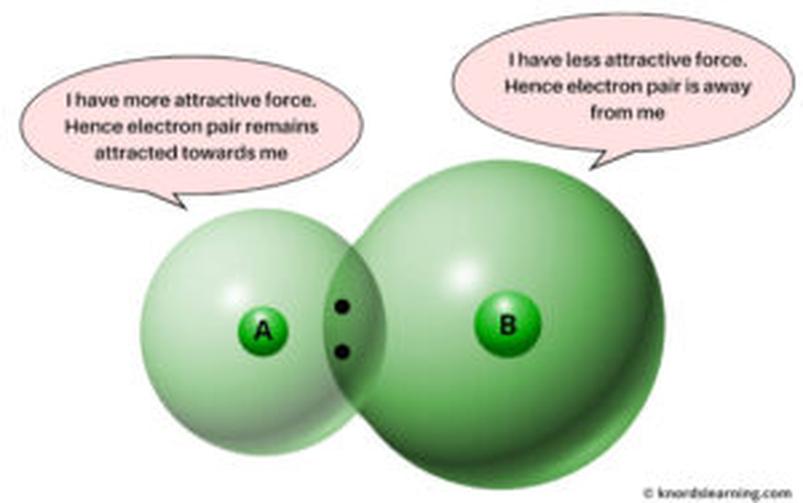
| Factor | Across Period (Left to Right) | Down Group (Top to Bottom) |
|---|---|---|
| Nuclear Charge | Increases → Stronger pull | Constant per period |
| Atomic Radius | Decreases → Electrons closer | Increases → Electrons farther |
| Electron Shells | Same number → Less shielding | More shells → More shielding |
| Electron Shell Completion | Closer to full → More attraction | Less impact ↑ because outer shells added |
Key Takeaways
- Electronegativity rises left to right due to increased nuclear charge and smaller atomic size.
- Moving up a group increases electronegativity as atoms have fewer shells, reducing distance and shielding.
- Atoms closer to full outer shells show greater desire to attract electrons.
- Combining these factors explains the consistent trend on the periodic table.
Why Does Electronegativity Increase Across and Up the Periodic Table?
Electronegativity increases across a period and up a group because the nucleus’ positive pull on electrons strengthens as more protons are packed in, pulling electrons closer and making atoms smaller. Fewer electron layers also mean that electrons sit nearer to the nucleus, increasing attraction. Sounds simple, right? But there’s a fascinating science story behind this trend that’s as structured as the periodic table itself.
Let’s dig deep into the atomic party where protons, electrons, and electron layers dance to the rhythms of physics. By understanding why electronegativity climbs across and upward, you unlock a key chemical insight that fuels everything from bonding to reactivity. Ready to dive into the nitty-gritty? Let’s do it.
Protons: The Positively Charged Managers of the Atom
Imagine the nucleus as a bustling office and protons as managers who pull in new recruits—electrons—with bright bonuses called positive charge. The more managers (protons) at the desk, the stronger the pull on the electrons wandering around. So when you move across a period (left to right), each new element stacks on another proton.
- More protons = stronger positive charge in the nucleus.
- Stronger charge means better electron attraction.
This tighter grip on electrons explains why elements on the right side of the periodic table (like fluorine and oxygen) are electronegativity champions—they have plenty of protons coaxing electrons closer. So rather than electrons lazing far out, they’re pulled in tighter, increasing electronegativity.
Atomic Shrinkage: Smaller Size, Stronger Grip
Here’s where atomic size plays a starring role. As protons pile up, their charge pulls electrons inward, shrinking the atom. This atomic shrinkage hugely impacts electronegativity because electrons that are physically closer to the nucleus feel a stronger pull. Distance matters; it’s like trying to win a tug-of-war—the closer you are, the stronger the pull.
- Across a period, atoms shrink because the nucleus pulls electrons inward.
- The smaller the atom, the closer the valence electrons are to the nucleus.
- This close proximity means electrons experience a stronger attractive force.
This explains why electronegativity goes up across a period—atoms aren’t just adding protons; they’re pulling electrons tighter, boosting their desire to attract more electrons. Think of it as an ever-shrinking dance floor, where the partners get closer and grip tighter.
The Layer Cake of Electrons: Why Going Down Means Loosening the Grip
But going down a group (column) in the periodic table tells a different tale. Each step down adds a whole new “layer” of electrons—a new electron shell. Imagine these as concentric orbits, like the rings of an onion. More layers push the outer electrons further from the nucleus.
- More electron shells = greater atomic radius (bigger atom).
- Greater distance between nucleus and valence electrons weakens pull.
- Thus electronegativity decreases as you go down a group.
So, why does electronegativity drop going down the table? Simply, the nucleus is still pulling, but the outer electrons are far from the dance floor—way too far for a strong pull. The “shielding” effect of inner layers also buffers the positive charge, further weakening the nucleus’ grip.
Almost Full or Barely Full? Electron Shells’ Influence on Desire
The number of electrons in the outer shell also affects electronegativity. Atoms get more desperate to attract electrons when they are close to filling their shell—like a nearly booked hotel desperately wanting just one more guest.
- Going left to right, atoms approach a full valence shell.
- Atoms near a full shell pull electrons harder to complete their “squad.”
- Fewer layers up the group mean valence electrons sit close, boosting pull.
This explains why electronegativity jumps across periods and up groups: the closer an atom is to a full outer shell and the fewer layers it needs to worry about, the more fiercely it attracts electrons. It’s a win-win cocktail of nuclear charge, atomic size, and electron configuration.
Putting It All Together: The Electronegative Climb
| Factor | Effect Across a Period (Left to Right) | Effect Down a Group (Top to Bottom) |
|---|---|---|
| Number of Protons | Increases; nucleus pulls electrons stronger. | Increases, but shielded by added layers. |
| Atomic Size | Decreases; electrons closer to nucleus. | Increases; electrons farther from nucleus. |
| Electron Shells | Same number; closer to full shells. | More shells; electrons shielded. |
| Electronegativity | Increases due to stronger pull and smaller size. | Decreases due to shielding and electron distance. |
This matrix neatly sums up why electronegativity isn’t random but follows logical patterns rooted in physics and atomic structure. It also shows how two competing effects—nuclear charge vs. electron shielding—explain the trends convincingly.
Why Does This Matter?
Electronegativity dictates how atoms interact, bond, and react.
Elements with high electronegativity, like chlorine or oxygen, are electron hogs in chemical bonds. Low electronegativity atoms, like sodium, give away electrons easily. Understanding these trends explains the behavior of molecules, from salty ocean waves (NaCl) to DNA’s life-sustaining structure.
Beautiful, isn’t it? The periodic table isn’t just a chart; it’s a map of electronegativity’s rise and fall, guiding chemists and students alike in predicting chemical drama at the atomic level.
Final Thought: Can You Predict Electronegativity?
Next time you look at the periodic table, ask yourself: “Why does fluorine pull electrons more than lithium?” or “Why do elements at the top hold onto their electrons like a tight handshake?” Understanding these forces lets you predict, explain, and master chemistry with clarity.
So whether you’re packing groceries or unraveling molecular mysteries, electronegativity’s upward and rightward hike is your trusty guide. Remember, more protons close to home mean a stronger pull, fewer layers mean less shielding—that’s the electronegativity dance, choreographed perfectly across the periodic table.
Why does electronegativity increase across a period from left to right?
As you move across a period, the number of protons rises. More protons mean a stronger positive charge. This stronger charge pulls electrons closer, increasing electronegativity.
How does atomic size affect electronegativity across the periodic table?
Atoms get smaller from left to right across a period. Smaller atoms hold electrons tighter because the nucleus is closer to the outer electrons. This boosts electronegativity.
Why does electronegativity decrease down a group?
Going down a group adds electron layers, which increase atomic size. With electrons farther from the nucleus, the attraction weakens, lowering electronegativity.
What role does electron shell filling play in electronegativity trends?
Atoms near a full outer shell attract more electrons to complete those shells. This desire grows left to right and top to bottom, affecting electronegativity values.
How does the distance between the nucleus and electrons influence electronegativity?
Electrons closer to the nucleus feel a stronger pull. Smaller atoms have less distance between the nucleus and electrons, making their electronegativity higher.




Leave a Comment