Why is the Ionization Energy of Magnesium Greater than That of Aluminum?
The ionization energy of magnesium (Mg) is greater than that of aluminum (Al) primarily because removing an electron from Mg’s fully filled 3s2 orbital requires more energy than removing one from Al’s singly occupied 3p1 orbital. This difference arises from electron configuration stability, orbital penetration effects, and effective nuclear attraction.
1. Electron Configuration and Stability
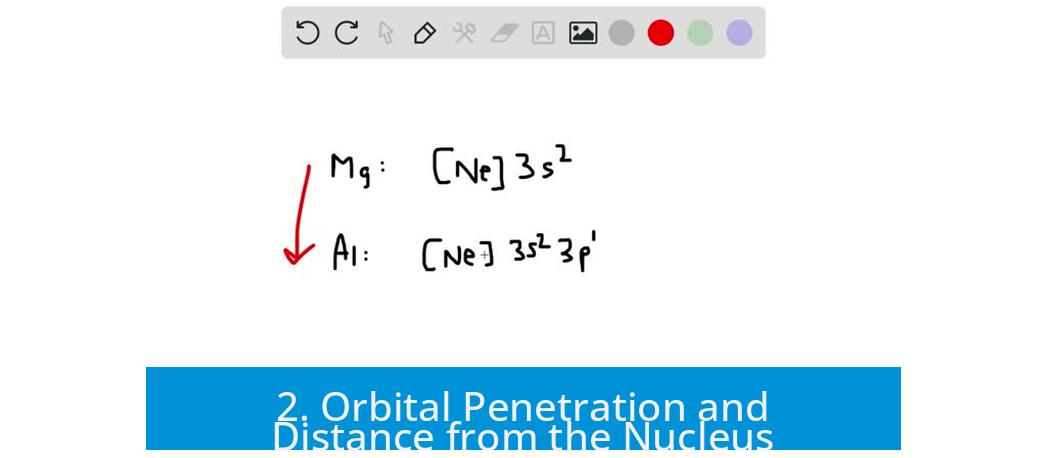
Magnesium has the electron configuration [Ne] 3s2, indicating two paired electrons occupy the 3s orbital. Aluminum, on the other hand, has [Ne] 3s2 3p1, with a single electron in the 3p orbital.
- Removing one electron from Mg means disrupting a stable, fully paired 3s2 configuration, producing 3s1.
- This leads to loss of electron pairing and increased electron repulsion in the remaining electron.
- By contrast, removing the lone 3p electron in Al simplifies the configuration to 3s2, a more stable, fully filled 3s orbital.
Half-filled or fully filled orbitals are energetically more favorable due to symmetrical electron distribution and minimized repulsions. Therefore, extracting an electron from a fully filled 3s2 (Mg) is harder than from a partially filled 3p1 (Al) state.
2. Orbital Penetration and Distance from the Nucleus
Electrons in s orbitals penetrate closer to the nucleus than those in p orbitals because s orbitals have electron density at the nucleus, whereas p orbitals have nodal planes with zero electron density at the nucleus.
- This higher penetration means 3s electrons in Mg feel a stronger effective nuclear charge, making them more tightly bound.
- Although Al has one more proton in its nucleus, its 3p valence electron is, on average, farther from the nucleus.
- As a result, the 3p electron in Al is less tightly held despite the increased nuclear charge.
A practical analogy is seen in potassium (K) and calcium (Ca), where 4s electrons fill before 3d because 4s electrons are closer and more stable due to greater nuclear penetration.
3. Stability of Orbital Configurations and Ion Formation
Removing an electron affects the stability of the resulting ion. For Mg, shedding an electron disrupts a full 3s orbital, reducing stability. In Al’s case, electron removal leads to a closed 3s shell, which is more stable.
Such differences influence ionization energies significantly. The formation of a half-filled 3s1 configuration in Mg is less energetically favorable compared to the formation of a fully filled 3s2 configuration in Al after electron removal.
4. Theoretical and Conceptual Considerations

Ionization energies generally increase across a period, correlating inversely with atomic radius. However, electronic configurations can create exceptions.
- In Mg and Al, the change in orbital type (3s vs. 3p) overrides simple atomic size trends.
- Heuristic orbital models capture this by focusing on where electrons reside and how stable the ions become after electron loss.
- Calculations confirm that ionization energy depends heavily on electron arrangements and effective nuclear charge experienced in different orbitals.
5. Summary of Key Physical Principles
| Factor | Magnesium (Mg) | Aluminum (Al) |
|---|---|---|
| Electron to Remove | 3s electron from filled 3s2 orbital | 3p electron from singly occupied 3p1 orbital |
| Orbital Penetration | High (s orbital) | Lower (p orbital) |
| Electron-Nucleus Distance | Closer penetration; more tightly bound | Farther; less tightly bound |
| Stability Effects | Loss of paired electron stability; less favorable | Moves to fully filled 3s2; more favorable |
| Net Ionization Energy | Higher due to stable filled subshell | Lower due to less stable 3p electron |
Key Takeaways
- Mg’s electrons occupy a fully filled 3s orbital with paired electrons; Al’s valence electron is in a singly occupied 3p orbital.
- 3s electrons penetrate closer to the nucleus and are held more tightly than 3p electrons.
- Removing a 3s electron from Mg disrupts a stable electron pairing; removing a 3p electron from Al yields a stable 3s2 configuration.
- The effective nuclear charge felt by electrons and orbital penetration influence ionization energies more than just nuclear charge or atomic radius.
- Thus, Mg has a higher first ionization energy than Al despite having fewer protons.
Why is the Ionization Energy of Magnesium Greater than That of Aluminum?
Simply put, the ionization energy of magnesium (Mg) is greater than that of aluminum (Al) because Mg’s valence electrons are in a stable, fully filled 3s orbital, while Al’s valence electron is in a higher energy, less tightly held 3p orbital. This difference in electron configuration and orbital nature means it takes more energy to remove an electron from Mg than from Al.
At first glance, this might seem counterintuitive. Aluminum has a higher nuclear charge (13 protons) compared to magnesium’s 12. You might expect Al to hold its electrons more tightly, demanding more energy to strip one away. However, the twist lies in where the electrons sit within the atom and how stable their configurations are.
Electron Configuration: It’s All About the Orbitals
Magnesium’s electron configuration ends with 3s2. Both electrons in the 3s orbital are paired up. Aluminum, on the other hand, ends with 3s2 3p1. That lone electron in 3p makes all the difference.
Removing an electron from Al means taking away the single 3p electron, leaving a filled 3s2 shell behind—very stable. For Mg, removing one electron breaks the paired set in the 3s shell, producing a less stable 3s1 state. Paired electrons enjoy a cozy neighborhood; losing one disrupts that comfort, making Mg resist electron removal more strongly.
This concept might sound familiar if you’ve heard that half-filled or fully filled orbitals offer extra stability. Indeed, the half-filled 3s1 state in Mg after ionization is actually less stable than the full 3s2; thus, ionization requires more energy.
So the energy balance tips toward easier ionization in Al despite its higher nuclear charge.
Orbital Penetration: How Close Do Electrons Get?
Electrons in s orbitals (like Mg’s 3s electrons) do more than just hang out further in space. They *penetrate* closer to the nucleus than those in p orbitals (like Al’s 3p electron). What does this mean? It means s-electrons experience a stronger pull from the nucleus.
Imagine two electrons, one lounging near the nucleus and another chilling farther out in the outskirts. The one lounging closer feels a stronger attraction — so it’s tougher to kick them out.
Al’s 3p electron is less tightly held because p orbitals have nodal planes, places where electron density drops to zero right at the nucleus. This means the 3p electron in aluminum is generally farther away, held more loosely despite the atom’s greater nuclear charge. Magnesium’s paired 3s electrons don’t enjoy this luxury — they live closer to the nucleus so they’re held more tightly.
Stability Makes All the Difference
The completely filled 3s orbital in Mg is more stable than the partially filled 3p orbital in Al. This difference is like the peace of a fully boarded house compared to a half-lived-in one; the former resists change more.
Here’s the kicker: removing an electron from Al actually increases its electronic stability by filling the 3s shell completely. Mg, on the other hand, faces a drop in stability after ionization.
It’s a subtle effect but a powerful one. Chemists often say “electron configuration trumps atomic size” in explaining these dips in ionization energies across periods. The so-called “exceptions” to the rule happen when changing electron orbitals alters the balance of orbital stability.
Beyond Atomic Radius: The Real Story
Common wisdom tells us ionization energy rises as atomic radius shrinks because electrons are closer to the nucleus and harder to remove. However, the Mg-Al case teaches a valuable lesson in chemistry’s complexity: you can’t judge ionization energies by size alone.
Electron configuration and orbital penetration add nuance. For instance, Mg and Al sit side-by-side in the periodic table’s third period. Still, Mg’s electrons have a tighter grip on their s orbitals, despite Al’s heavier nucleus.
Electron repulsion also plays a role. The paired electrons in Mg’s 3s orbital repel each other. When one is removed, repulsion lessens, so the system “prefers” to lose an electron somewhat reluctantly. Removing the lone 3p electron in Al involves less repulsion cost, making it energetically easier.
How Does This Affect Real-Life Chemistry?
This difference in ionization energies impacts how magnesium and aluminum behave in reactions. Magnesium tends to lose its electrons less readily compared to aluminum, leading to subtle differences in their reactivity and the types of compounds they form.
For example, magnesium metal resists corrosion a bit better than aluminum, partly due to its ionization characteristics. And in materials science, these properties influence how alloys with Mg and Al behave under stress and in different environments.
What Can You Take Away?
- Electron configuration matters more than just atomic number. It determines how tightly electrons are bound.
- Orbital penetration is key. Electrons in s orbitals hug the nucleus tighter than those in p orbitals.
- Paired electrons in fully filled orbitals resist removal more. It’s about stability, not just charge.
- Higher nuclear charge doesn’t always mean higher ionization energy. The orbital holding the electron plays a big role.
So the next time someone tells you that bigger nuclear charge always makes it harder to remove electrons, you can smile knowingly and say: “Not when orbital configurations have a say!”
In the fascinating dance of electrons, the details count. Magnesium holds on tight because it simply can. And aluminum? It lets go a little easier.
Why does magnesium have a higher ionization energy than aluminum despite aluminum having more protons?
Although Al has more protons, its outer electron is in a 3p orbital, which is farther from the nucleus and less tightly held than Mg’s 3s electrons. This makes Mg’s electrons harder to remove.
How does electron configuration affect the ionization energy difference between Mg and Al?
Mg has a filled 3s2 orbital, making electron removal less favorable because it disrupts paired stability. Al has an unpaired electron in 3p1, which is easier to remove, lowering its ionization energy.
Why is removing an electron from Al’s 3p orbital more favorable than from Mg’s 3s orbital?
Removing an electron from Al goes from 3p1 to a stable 3s2 configuration. For Mg, removing an electron changes 3s2 to 3s1, which reduces stability and increases electron repulsion, requiring more energy.
What role does orbital penetration play in the ionization energy difference of Mg and Al?
S orbitals penetrate closer to the nucleus than p orbitals. Mg’s electrons in the 3s orbital are held more tightly, while Al’s outer electron in the 3p orbital experiences less nuclear attraction.
Does atomic radius explain why Mg has higher ionization energy than Al?
Atomic radius alone doesn’t explain this. Ionization energy depends more on electron configuration and orbital type, with s orbital electrons being more tightly bound despite similar or larger atomic sizes in Al.




Leave a Comment